
园艺学报 ›› 2021, Vol. 48 ›› Issue (6): 1079-1093.doi: 10.16420/j.issn.0513-353x.2020-0902
马俊杰1, 宋丽娜1, 李乐1, 马晓春1, 靳磊1, 徐伟荣2,3,4,*( )
)
收稿日期:2021-03-16
修回日期:2021-05-11
出版日期:2021-06-25
发布日期:2021-07-07
通讯作者:
徐伟荣
E-mail:xuwr@nxu.edu.cn
基金资助:
MA Junjie1, SONG Lina1, LI Le1, MA Xiaochun1, JIN Lei1, XU Weirong2,3,4,*( )
)
Received:2021-03-16
Revised:2021-05-11
Online:2021-06-25
Published:2021-07-07
Contact:
XU Weirong
E-mail:xuwr@nxu.edu.cn
摘要:
对山葡萄(Vitis amurensis)‘左山-1'中的1个类钙调素磷酸酶B亚基蛋白基因VaCBL6进行克隆及功能分析。VaCBL6位于第4条染色体,开放阅读框为777 bp,编码258个氨基酸;结构域分析表明,该蛋白包含4个EF-hand与1个跨膜结构域;蛋白进化树分析表明,VaCBL6与欧洲葡萄VvCBL9和VvCBL6相似性较高,分别为98.84%、94.96%。VaCBL6编码蛋白定位于细胞核与细胞膜。VaCBL6在根与卷须中有较高的表达,且其表达受低温、干旱、NaCl和ABA的诱导。基于酵母体系的转录激活分析表明,VaCBL6无转录激活活性。100 ~ 125 μmol · L -1 NaCl和0.25 ~ 1.25 μmol · L -1 ABA处理均抑制了VaCBL6转基因拟南芥种子的萌发,且转化拟南芥幼苗对NaCl与ABA的敏感性提高。以上结果说明VaCBL6在植物的逆境胁迫应答中具有重要作用。
中图分类号:
马俊杰, 宋丽娜, 李乐, 马晓春, 靳磊, 徐伟荣. 山葡萄VaCBL6参与非生物胁迫和ABA途径的响应[J]. 园艺学报, 2021, 48(6): 1079-1093.
MA Junjie, SONG Lina, LI Le, MA Xiaochun, JIN Lei, XU Weirong. VaCBL6 from Vitis amurensis Involved in Abiotic Stress Response and ABA-mediated Pathway[J]. Acta Horticulturae Sinica, 2021, 48(6): 1079-1093.
| 引物 Primer | 引物序列(5′-3′) Primer sequence |
|---|---|
| qPCR-CBL6-F | TGCTGCGACTTCCACAAA |
| qPCR-CBL6-R | CTCATACAGTGCCTCCACTTC |
| RT-VvActin-F | CTATCCTTCGTCTTGACCTTGCTG |
| RT-VvActin-R | AGTGGTGAACATGTAACCCCTCTC |
| Semi-CBL6-F | GGAAGAGCTTCAATTGGCGT |
| Semi-CBL6-R | ACTGTGGTGATGTCCTTCAAAT |
| AtActin2-RT-F | TGAGCAAAGAAATCACAGCACT |
| AtActin2-RT-R | CCTGGACCTGCCTCATCATAC |
| VaCBL6-ORF-F | ATGAGTTCTTGGCAGGGAACGGCG |
| VaCBL6-ORF-R | TCATTCTTCAACCTCAGTATTGAAAACAAAGC |
| 221-VaCBL6-F | GAGAGAACACGGGGGACTCTAGAATGAGTTCTTGGCAGGGAACGGCG |
| 221-VaCBL6-R | TTACCCATGGTACCCCGCTCGAGTTCTTCAACCTCAGTATTGAAAACAAAGC |
| BD-CBL6-F | atggccatggaggccgaattcATGAGTTCTTGGCAGGGAACG |
| BD-CBL6-R | ccgctgcaggtcgacggatccTCATTCTTCAACCTCAGTATTGAAAAC |
| Entry-VaCBL6-F | AAAAAAgCAggCTTTgACTTTATgAgTTCTTggCAgggAACg |
| Entry-VaCBL6-R | AAAgCTgggTCTAgAgACTTTCCTTCTTCAACCTCAgTATTgA |
表1 各种载体构建及VaCBL6 表达检测引物
Table 1 Primers of vector generation and the expression of VaCBL6
| 引物 Primer | 引物序列(5′-3′) Primer sequence |
|---|---|
| qPCR-CBL6-F | TGCTGCGACTTCCACAAA |
| qPCR-CBL6-R | CTCATACAGTGCCTCCACTTC |
| RT-VvActin-F | CTATCCTTCGTCTTGACCTTGCTG |
| RT-VvActin-R | AGTGGTGAACATGTAACCCCTCTC |
| Semi-CBL6-F | GGAAGAGCTTCAATTGGCGT |
| Semi-CBL6-R | ACTGTGGTGATGTCCTTCAAAT |
| AtActin2-RT-F | TGAGCAAAGAAATCACAGCACT |
| AtActin2-RT-R | CCTGGACCTGCCTCATCATAC |
| VaCBL6-ORF-F | ATGAGTTCTTGGCAGGGAACGGCG |
| VaCBL6-ORF-R | TCATTCTTCAACCTCAGTATTGAAAACAAAGC |
| 221-VaCBL6-F | GAGAGAACACGGGGGACTCTAGAATGAGTTCTTGGCAGGGAACGGCG |
| 221-VaCBL6-R | TTACCCATGGTACCCCGCTCGAGTTCTTCAACCTCAGTATTGAAAACAAAGC |
| BD-CBL6-F | atggccatggaggccgaattcATGAGTTCTTGGCAGGGAACG |
| BD-CBL6-R | ccgctgcaggtcgacggatccTCATTCTTCAACCTCAGTATTGAAAAC |
| Entry-VaCBL6-F | AAAAAAgCAggCTTTgACTTTATgAgTTCTTggCAgggAACg |
| Entry-VaCBL6-R | AAAgCTgggTCTAgAgACTTTCCTTCTTCAACCTCAgTATTgA |
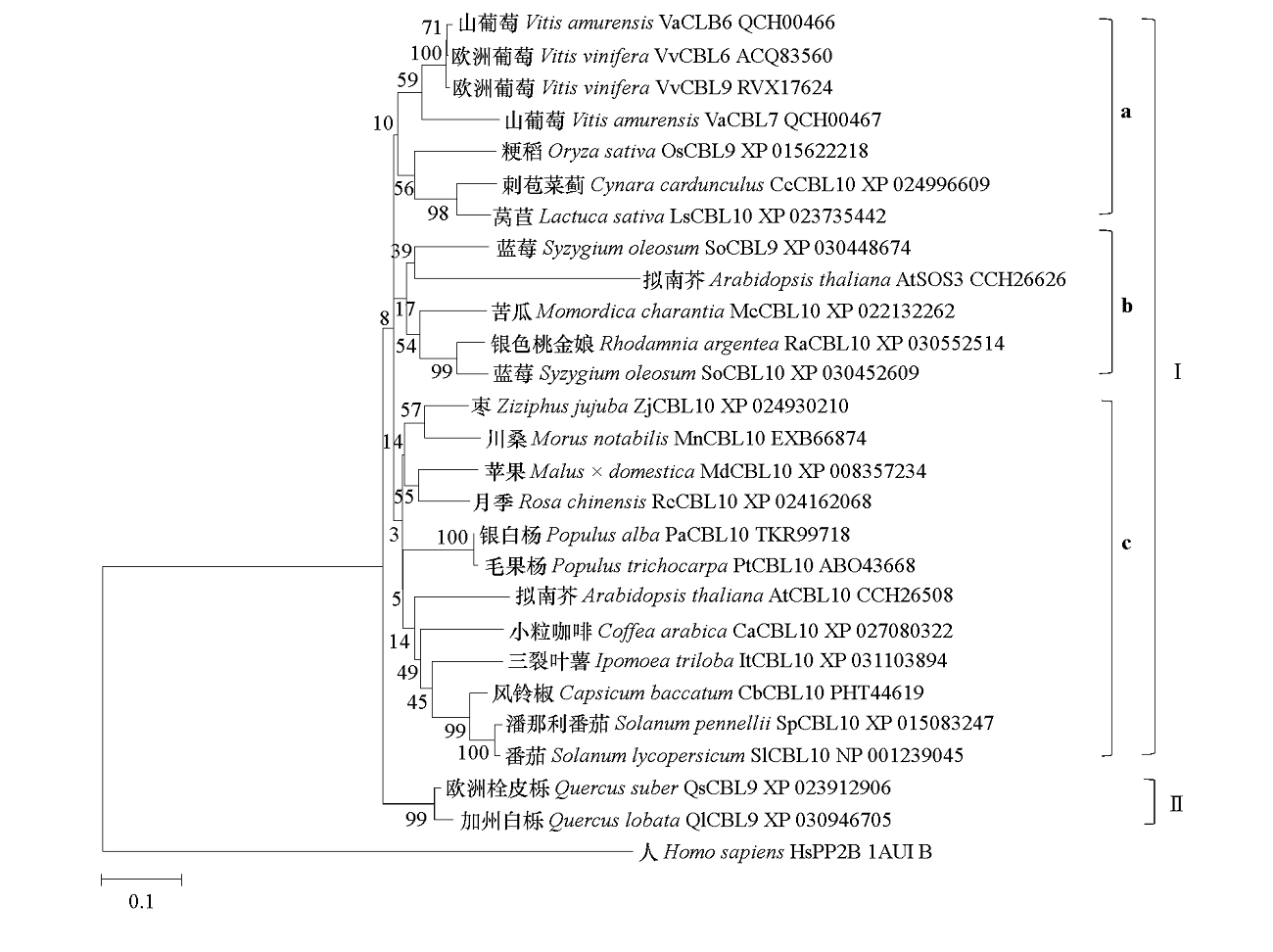
图2 VaCBL6和其他物种CBL氨基酸序列的聚类分析 采用MEGA 7.0的邻接法构建系统进化树。相关分类群聚集在一起的分支旁边显示百分比(%),分支长度以每个点的替换数量来度量。自展值为1 000。
Fig. 2 Phylogenetic relationships of Vitis amurensis VaCBL6 with CBLs from other plant species Protein sequences were aligned to construct a phylogenetic tree using the Neighbor-Joining(NJ)method by MEGA 7.0 software. The percentage of trees with related clusters were displayed next to the branches,and the phylogenetic tree was drawn proportionally with branch lengths measured in the number of substitutions per site. Bootstrap value was 1 000.
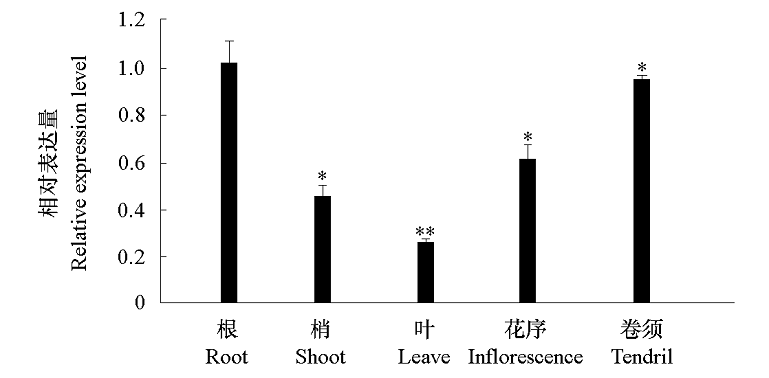
图4 山葡萄VaCBL6 在不同组织中的表达特性 *,** 表示VaCBL6的表达量与根(表达量为1)相比差异显著(* α = 0.05,** α = 0.01)。
Fig. 4 Expression pattern of VaCBL6 in various tissues of Vitis amurensis *,** indicated significant differences compared with the expression level of root(expression level is 1)(* α = 0.05,** α = 0.01).
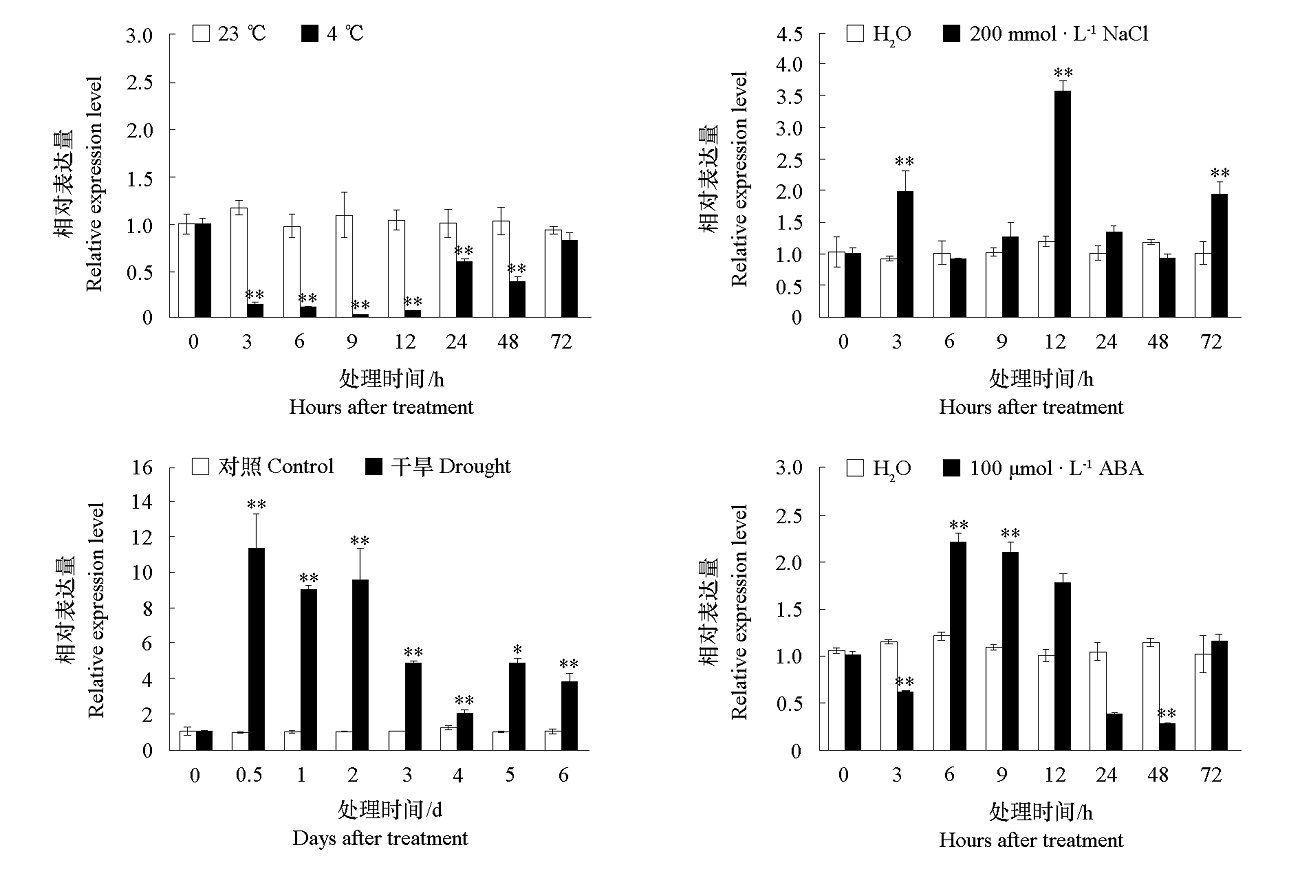
图5 山葡萄在非生物胁迫与ABA处理下叶片中VaCBL6的表达分析 * 和 ** 代表与同期对照相比差异显著(* α = 0.05,** α = 0.01)。
Fig. 5 Expression pattern of VaCBL6 in grape leaves under abiotic stress and ABA treatment * and ** indicated significant differences compared with control groups(* α = 0.05,** α = 0.01).
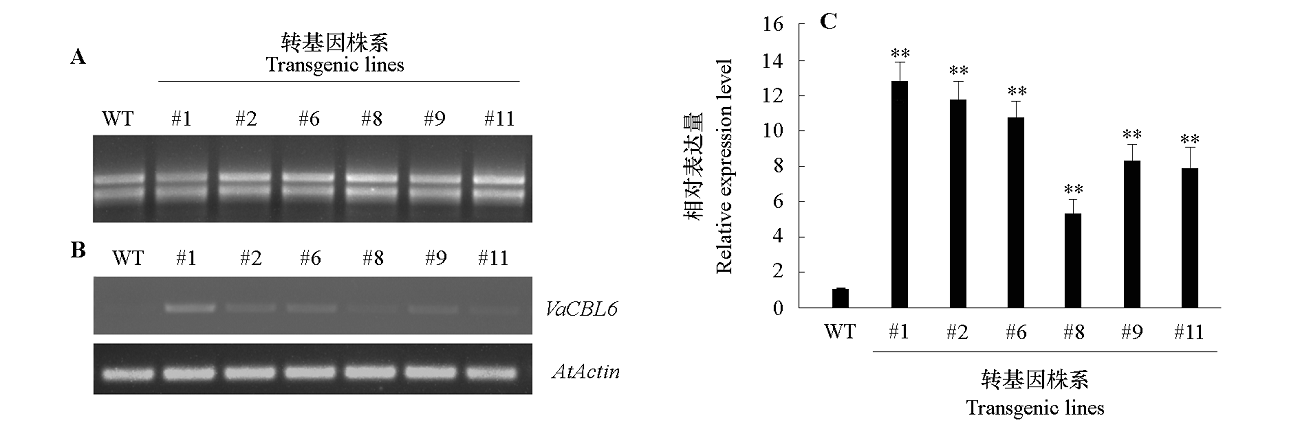
图8 VaCBL6过表达拟南芥株系的鉴定 A:RNA分离电泳;B:半定量RT-PCR分析;C:qRT-PCR分析。WT:野生型。** α = 0.01。
Fig. 8 Identification of VaCBL6-overexpressing lines in Arabidopsis thaliana A:Agarose gel electrophoresis of RNA;B:Expression of VaCBL6 analyzed by semi quantitative RT-PCR;C:Expression level of VaCBL6 analyzed by qRT-PCR. WT:Wild type. ** α = 0.01.

图9 VaCBL6过量表达拟南芥株系(#1、#2和#6)在含不同浓度NaCl的1/2 MS培养基上的生长情况 WT:野生型;#1,#2和#6:转基因株系。下同。
Fig. 9 Phenotypes of Arabidopsis thaliana overexpressing VaCBL6 lines(#1,#2 and #6)on 1/2 MS medium supplemented with different concentrations of NaCl WT:Wild type;#1,#2 and #6:35S::VaCBL6 transgenic lines. The same below.
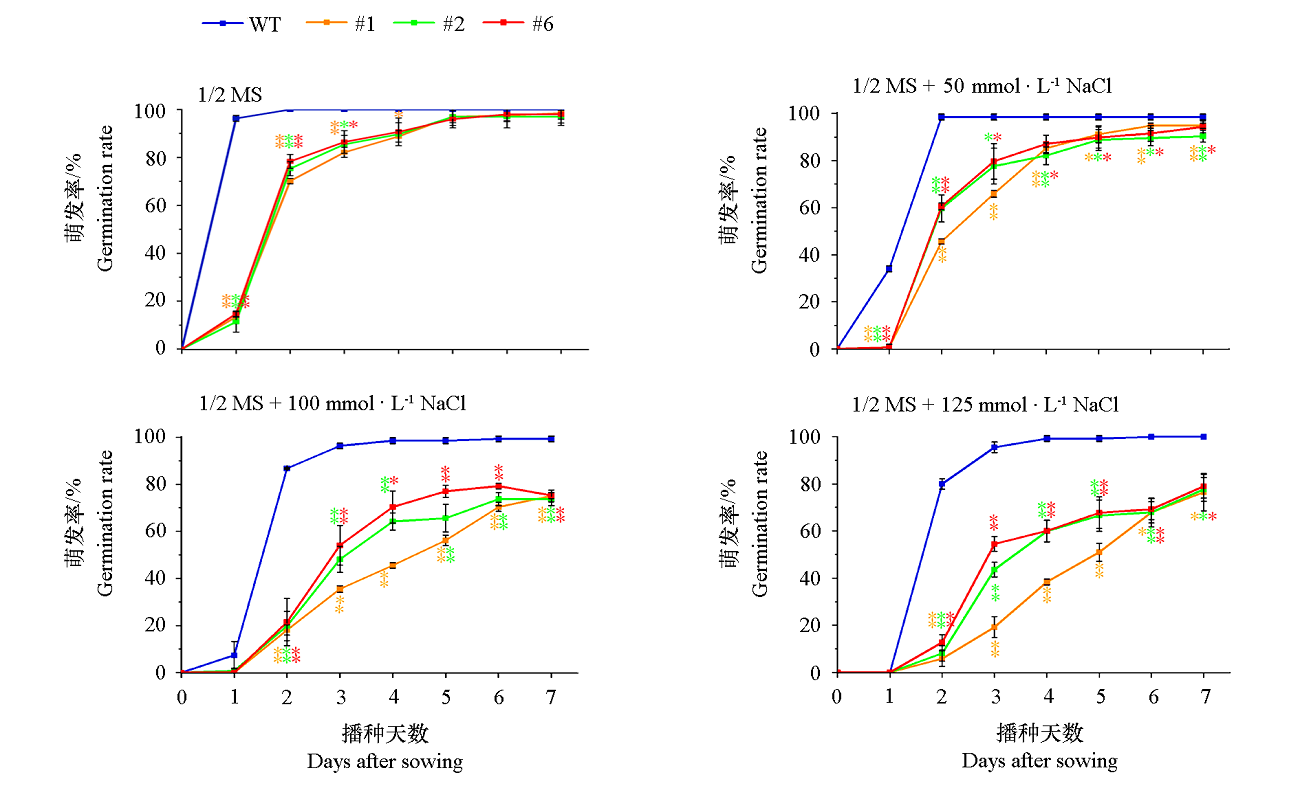
图10 VaCBL6过量表达拟南芥株系(#1、#2和 #6)在含不同浓度NaCl的1/2 MS培养基上0 ~ 7 d的萌发率
Fig. 10 Germination rates of Arabidopsis thaliana overexpressing VaCBL6 lines(#1,#2 and #6)under different concentrations of NaCl at 0-7 d
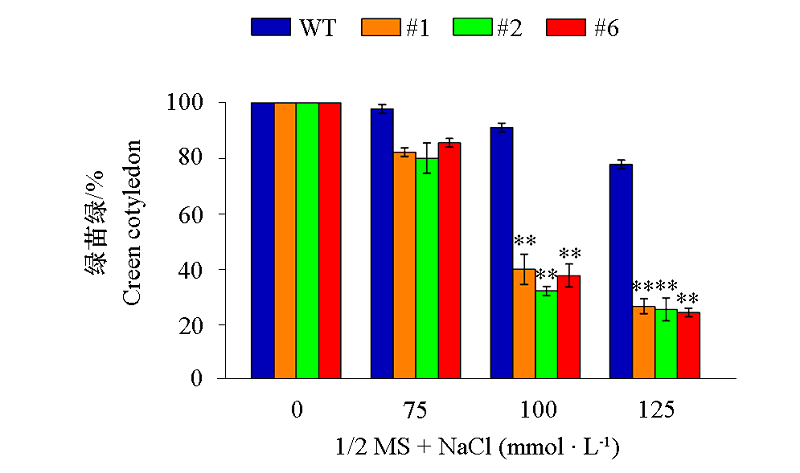
图11 VaCBL6过量表达拟南芥株系(#1、#2和#6)在含不同浓度NaCl的1/2 MS培养基上播种10 d时的绿苗率
Fig. 11 Germination rates of green cotyledon of seedlings of Arabidopsis thaliana overexpressing VaCBL6 lines (#1,#2 and #6)grown for 10 days under different concentrations of NaCl

图12 VaCBL6过表达拟南芥株系(#1、#2和#6)在含不同浓度ABA的1/2 MS培养基上的生长情况
Fig. 12 Phenotypes of Arabidopsis thaliana overexpressing VaCBL6 lines(#1,#2 and #6) on 1/2 MS medium supplemented with different concentrations of ABA
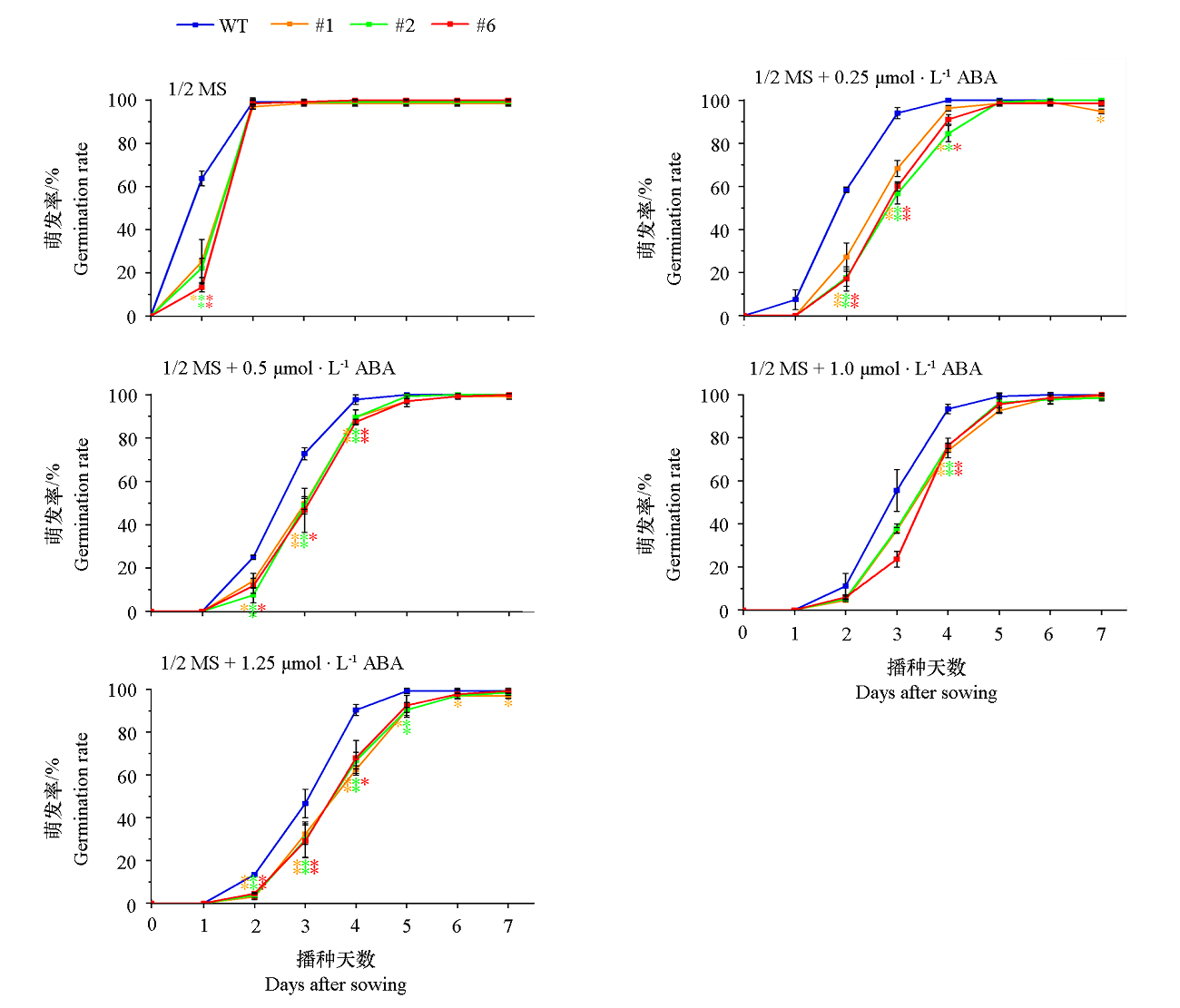
图13 VaCBL6过表达拟南芥株系(#1、#2和#6)在含不同浓度ABA的1/2 MS培养基上0 ~ 7 d的萌发率
Fig. 13 Germination rates of Arabidopsis thaliana overexpressing VaCBL6 lines(#1,#2 and #6) under different concentrations of ABA at 0-7 d
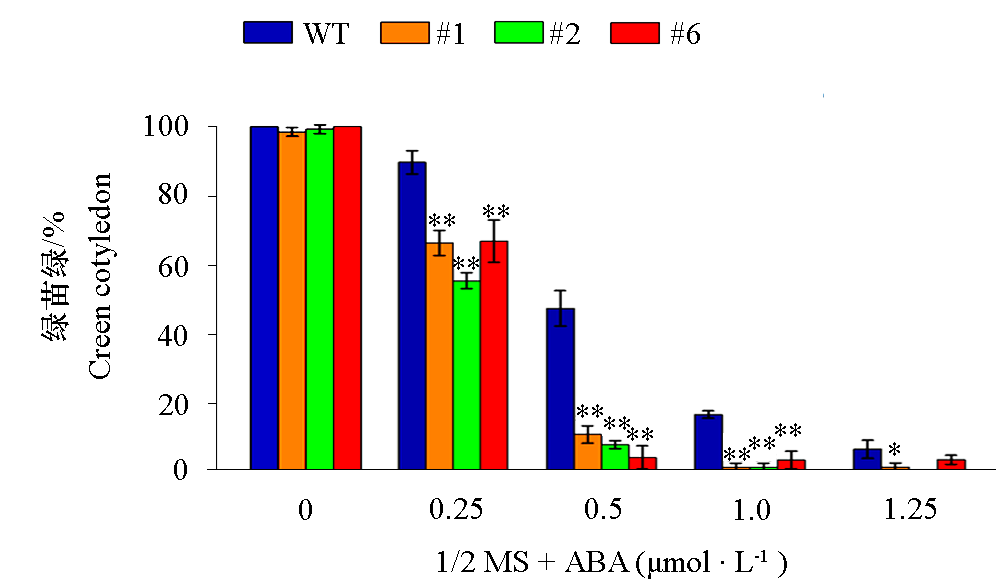
图14 VaCBL6过表达拟南芥株系(#1、#2和#6)在含不同浓度ABA的1/2 MS培养基上播种10 d时的绿苗率
Fig. 14 Germination rates of green cotyledon of seedlings of Arabidopsis thaliana overexpressing VaCBL6 lines (#1,#2 and #6)grown for 10 d under different concentrations of ABA
| [1] |
Albrecht V, Ritz O, Linder S, Harter K, Kudla J. 2001. The NAF domain defines a novel protein-protein interaction module conserved in Ca2+ regulated kinases. The EMBO Journal, 20 (5):1051-1063.
doi: 10.1093/emboj/20.5.1051 URL |
| [2] |
Batistič O, Waadt R, Steinhorst L, Held K, Kudla J. 2010. CBL mediated targeting of CIPKs facilitates the decoding of calcium signals emanating from distinct cellular stores. The Plant Journal, 61 (2):211-222.
doi: 10.1111/j.1365-313X.2009.04045.x URL |
| [3] | Batistič O, Kudla J. 2012. Analysis of calcium signaling pathways in plants. Biochimica et Biophysica Acta(BBA)-General Subjects, 1820 (8):1283-1293. |
| [4] |
Carmona M J, Cubas P, Calonje M, Martinez-Zapater J M. 2007. Flowering transition in grapevine(Vitis vinifera L.). Canadian Journal of Botany, 85 (8):701-711.
doi: 10.1139/B07-059 URL |
| [5] | Chen Sha-sha, Lan Hai-yan. 2011. Signal transduction pathways in response to salt stress in plants. Plant Physiology Journal, 47 (2):119-128. (in Chinese) |
| 陈莎莎, 兰海燕. 2011. 植物对盐胁迫响应的信号转导途径. 植物生理学报, 47 (2):119-128. | |
| [6] |
Chen X, Gu Z, Xin D, Hao L, Liu C, Huang J, Ma B, Zhang H. 2011. Identification and characterization of putative CIPK genes in maize. Journal of Genetics and Genomics, 38 (2):77-87.
doi: 10.1016/j.jcg.2011.01.005 URL |
| [7] |
Clough S J, Bent A F. 1998. Floral dip:a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal, 16 (6):735-743.
doi: 10.1046/j.1365-313x.1998.00343.x URL |
| [8] | Dong Lian-hong, Shi Su-juan, Nuruzzaman Manik S. 2015. Advances in research of CBL family in plant. Journal of Nuclear Agricultural Sciences, 29 (5):892-898. (in Chinese) |
| 董连红, 史素娟, Nuruzzaman Manik S. 2015. 植物CBL基因家族的研究进展. 核农学报, 29 (5):892-898. | |
| [9] |
Gu Z, Ma B, Jiang Y, Chen Z, Su X, Zhang H. 2008. Expression analysis of the calcineurin B-like gene family in rice(Oryza sativa L.)under environmental stresses. Gene, 415 (1-2):1-12.
doi: 10.1016/j.gene.2008.02.011 URL |
| [10] |
Hepler P K. 2005. Calcium:a central regulator of plant growth and development. The Plant Cell, 17 (8):2142-2155.
doi: 10.1105/tpc.105.032508 URL |
| [11] |
Ishitani M, Liu J, Halfter U, Kim C S, Shi W, Zhu J K. 2000. SOS 3 function in plant salt tolerance requires N-myristoylation and calcium binding. The Plant Cell, 12:1667-1677.
doi: 10.1105/tpc.12.9.1667 URL |
| [12] |
Kolukisaoglu U, Weinl S, Blazevic D, Batistič O, Kudla J. 2004. Calcium sensors and their interacting protein kinases:genomics of the Arabidopsis and rice CBL-CIPK signaling networks. Plant Physiology, 134 (1):43-58.
doi: 10.1104/pp.103.033068 URL |
| [13] | Ling Q, Zeng Q, Wu J, Hu F, Li Q, Qi Y. 2019. Expression analysis of CBL1 and CBL6 genes in sugarcane under abiotic stress. Molecular Plant Breeding, 10 (1):1-10. |
| [14] |
Liu J, Zhu J K. 1998. A calcium sensor homolog required for plant salt tolerance. Science, 280:1943-1945.
doi: 10.1126/science.280.5371.1943 URL |
| [15] |
Liu P, Duan Y, Liu C, Xue Q, Guo J, Qi T, Kang Z, Guo J. 2018. The calcium sensor TaCBL4 and its interacting protein TaCIPK 5 are required for wheat resistance to stripe rust fungus. Journal of Experimental Botany, 69 (21):4443-4457.
doi: 10.1093/jxb/ery227 URL |
| [16] | Mullins M G, Bouquet A, Williams L E. 1992. Biology of the grapevine. UK: Cambridge University Press. |
| [17] | Qiu Q S, Guo Y, Dietrich M A, Schumaker K S, Zhu J K. 2002. Regulation of SOS1,a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana,by SOS2 and SOS3. Proceedings of the National Academy of Sciences, 99 (12):8436-8441. |
| [18] |
Quan R, Lin H, Mendoza I, Zhang Y, Cao W, Yang Y, Shang M, Chen S, Pardo J M, Guo Y. 2007. SCABP8/CBL10,a putative calcium sensor,interacts with the protein kinase SOS 2 to protect Arabidopsis shoots from salt stress. The Plant Cell, 19 (4):1415-1431.
doi: 10.1105/tpc.106.042291 URL |
| [19] |
Sánchez-Barrena M J, Martínez-Ripoll M, Zhu J K, Albert A. 2005. The structure of the Arabidopsis thaliana SOS3:molecular mechanism of sensing calcium for salt stress response. Journal of Molecular Biology, 345 (5):1253-1264.
doi: 10.1016/j.jmb.2004.11.025 URL |
| [20] |
Tang R J, Liu H, Yang Y, Yang L, Gao X S, Garcia V J, Luan S, Zhang H X. 2012. Tonoplast calcium sensors CBL2 and CBL 3 control plant growth and ion homeostasis through regulating V-ATPase activity in Arabidopsis. Cell Research, 22 (12):1650-1665.
doi: 10.1038/cr.2012.161 URL |
| [21] | Tang R J, Yang Y, Yang L, Liu H, Wang C T, Yu M M, Gao X S, Zhang H X. 2014. Poplar calcineurin B-like proteins PtCBL10A and PtCBL10B regulate shoot salt tolerance through interaction with PtSOS 2 in the vacuolar membrane. Plant Cell & Environment, 37 (3):573-588. |
| [22] |
Weinl S, Kudla J. 2009. The CBL-CIPK Ca 2+-decoding signaling network: function and perspectives. New Phytologist, 184 (3):517-528.
doi: 10.1111/nph.2009.184.issue-3 URL |
| [23] |
Wu F H, Shen S C, Lee L-Y, Lee S H, Chan M T, Lin C S. 2009. Tape- Arabidopsis Sandwich-a simpler Arabidopsis protoplast isolation method. Plant Methods, 5:16.
doi: 10.1186/1746-4811-5-16 URL |
| [24] |
Xu W, Shen W, Ma J, Ya R, Zheng Q, Wu N, Yu Q, Yao W, Zhang N, Zhang J. 2020. Role of an Amur grape CBL-interacting protein kinase VaCIPK02 in drought tolerance by modulating ABA signaling and ROS production. Environmental and Experimental Botany, 172:103999.
doi: 10.1016/j.envexpbot.2020.103999 URL |
| [25] |
Yu Y, Xia X, Yin W, Zhang H. 2007. Comparative genomic analysis of CIPK gene family in Arabidopsis and Populus. Plant Growth Regulation, 52 (2):101-110.
doi: 10.1007/s10725-007-9165-3 URL |
| [26] | Yu Yi-he, Li Xiu-zhen, Guo Da-long, Yang Ying-jun, Li Gui-rong, Li Xue-qiang, Zhang Guo-hai. 2016. Isolation and expression analysis of calcineurin B-like protein VvCBL4 in grapevines. Journal of Fruit Science, 33 (4):385-392. (in Chinese) |
| 余义和, 李秀珍, 郭大龙, 杨英军, 李桂荣, 李学强, 张国海. 2016. 葡萄类钙调磷酸酶B亚基蛋白基因 VvCBL4的克隆与表达分析. 果树学报, 33 (4):385-392. | |
| [27] |
Zhang H, Yin W, Xia X. 2008. Calcineurin B-Like family in Populus:comparative genome analysis and expression pattern under cold,drought and salt stress treatment. Plant Growth Regulation, 56 (2):129-140.
doi: 10.1007/s10725-008-9293-4 URL |
| [1] | 徐小萍, 曹清影, 蔡柔荻, 官庆栩, 张梓浩, 陈裕坤, 徐涵, 林玉玲, 赖钟雄. 龙眼miR408与DlLAC12克隆及其在球形胚发生和非生物胁迫下的表达分析[J]. 园艺学报, 2022, 49(9): 1866-1882. |
| [2] | 王沙, 张心慧, 赵玉洁, 李变变, 招雪晴, 沈雨, 董建梅, 苑兆和. 石榴花青苷合成相关基因PgMYB111的克隆与功能分析[J]. 园艺学报, 2022, 49(9): 1883-1894. |
| [3] | 贾鑫, 曾臻, 陈月, 冯慧, 吕英民, 赵世伟. 月季‘月月粉’RcDREB2A的克隆与表达分析[J]. 园艺学报, 2022, 49(9): 1945-1956. |
| [4] | 林元秘, 朱文姣, 陈敏, 薛春梅, 晋芳宇, 朱羽平, 蒋欣玥, 叶凌峰, 倪姝南伶, 杨清. miR396b负调控茄子对黄萎病的防御反应[J]. 园艺学报, 2022, 49(8): 1713-1722. |
| [5] | 邱子文, 刘林敏, 林永盛, 林晓洁, 李永裕, 吴少华, 杨超. 千层金MbEGS基因的克隆与功能分析[J]. 园艺学报, 2022, 49(8): 1747-1760. |
| [6] | 郑林, 王帅, 刘语诺, 杜美霞, 彭爱红, 何永睿, 陈善春, 邹修平. 柑橘响应黄龙病菌侵染的NAC基因的克隆及表达分析[J]. 园艺学报, 2022, 49(7): 1441-1457. |
| [7] | 马维峰, 李艳梅, 马宗桓, 陈佰鸿, 毛娟. 苹果POD家族基因的鉴定与MdPOD15的功能分析[J]. 园艺学报, 2022, 49(6): 1181-1199. |
| [8] | 刘瑶瑶, 吴严严, 石岩, 毛天宇, 包满珠, 张俊卫, 张杰. 垂枝与直枝梅花PmTAC1启动子序列差异与垂枝性状的关系初探[J]. 园艺学报, 2022, 49(6): 1327-1338. |
| [9] | 赵建荣, 杨圆, 秦改花, 刘春燕, 于晴, 贾波涛, 苏颖, 曹榛, 黎积誉. 石榴HAK/KUP/KT家族基因鉴定及钾转运功能分析[J]. 园艺学报, 2022, 49(4): 758-768. |
| [10] | 相立, 赵蕾, 王玫, 吕毅, 王艳芳, 沈向, 陈学森, 尹承苗, 毛志泉. 苹果MdWRKY74的克隆和功能分析[J]. 园艺学报, 2022, 49(3): 482-492. |
| [11] | 周至铭, 杨佳宝, 张程, 曾令露, 孟晚秋, 孙黎. 向日葵LACS家族鉴定及响应非生物胁迫表达分析[J]. 园艺学报, 2022, 49(2): 352-364. |
| [12] | 宋蒙飞, 查高辉, 陈劲枫, 娄群峰. 黄瓜株型性状分子基础研究进展[J]. 园艺学报, 2022, 49(12): 2683-2702. |
| [13] | 谢思艺, 周承哲, 朱晨, 詹冬梅, 陈兰, 吴祖春, 赖钟雄, 郭玉琼. 茶树CsTIFY家族全基因组鉴定及非生物胁迫和激素处理中主要基因表达分析[J]. 园艺学报, 2022, 49(1): 100-116. |
| [14] | 李茂福, 杨媛, 王华, 范又维, 孙佩, 金万梅. 月季自交不亲和性S-RNase的鉴定与分析[J]. 园艺学报, 2022, 49(1): 157-165. |
| [15] | 梁志乐, 汪宽鸿, 杨静, 祝彪, 朱祝军. 硫代葡萄糖苷在十字花科植物应对非生物胁迫中的作用[J]. 园艺学报, 2022, 49(1): 200-220. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司