
园艺学报 ›› 2022, Vol. 49 ›› Issue (9): 1866-1882.doi: 10.16420/j.issn.0513-353x.2021-0573
徐小萍1, 曹清影1, 蔡柔荻1, 官庆栩1, 张梓浩1, 陈裕坤1, 徐涵1,2, 林玉玲1, 赖钟雄1,*( )
)
收稿日期:2021-10-01
修回日期:2022-06-16
出版日期:2022-09-25
发布日期:2022-10-08
通讯作者:
赖钟雄
E-mail:laizx01@163.com
基金资助:
XU Xiaoping1, CAO Qingying1, CAI Roudi1, GUAN Qingxu1, ZHANG Zihao1, CHEN Yukun1, XU HAN1,2, LIN Yuling1, LAI Zhongxiong1,*( )
)
Received:2021-10-01
Revised:2022-06-16
Online:2022-09-25
Published:2022-10-08
Contact:
LAI Zhongxiong
E-mail:laizx01@163.com
摘要:
为探讨miR408及靶基因DlLAC12在龙眼球形体细胞胚诱导及不同非生物胁迫下的表达模式,采用miR-RACE PCR和Tail-PCR克隆获得pri-miR408 cDNA和转录起始位点、dlo-miR408 gDNA、靶基因DlLAC12 cDNA及启动子pro-MIR408序列。研究结果显示:dlo-pri-miR408 cDNA、gDNA全长均为706 bp,5′端转录起始位点为胞嘧啶(C)。DlLAC12 cDNA全长为1 725 bp,pro-MIR408全长为1 532 bp。pro-MIR408序列上存在ABA、GA3、JA等激素信号传导顺式作用元件和响应光、低温胁迫等相关元件。qRT-PCR结果显示,dlo-miR408-3p随外源添加的蔗糖浓度、Cu2+浓度及培养温度的变化呈现动态表达;而dlo-miR408-5p1对蔗糖不敏感,在铜离子失衡和低温时下调表达。dlo-miR408-3p与DlLAC12负调控模式能响应高浓度ABA(≥ 500 μmol · L-1)处理,而不同浓度GA3处理下二者表达模式一致。dlo-miR408-3p与DlLAC12在球形胚诱导不同天数呈现负调控,说明在球形胚诱导过程发挥作用。试验表明,dlo-miR408与靶基因DlLAC12可参与龙眼体胚发生与非生物胁迫响应。
中图分类号:
徐小萍, 曹清影, 蔡柔荻, 官庆栩, 张梓浩, 陈裕坤, 徐涵, 林玉玲, 赖钟雄. 龙眼miR408与DlLAC12克隆及其在球形胚发生和非生物胁迫下的表达分析[J]. 园艺学报, 2022, 49(9): 1866-1882.
XU Xiaoping, CAO Qingying, CAI Roudi, GUAN Qingxu, ZHANG Zihao, CHEN Yukun, XU HAN, LIN Yuling, LAI Zhongxiong. Gene Cloning and Expression Analysis of miR408 and Its Target DlLAC12 in Globular Embryo Development and Abiotic Stress in Dimocarpus longan[J]. Acta Horticulturae Sinica, 2022, 49(9): 1866-1882.
| 用途 Purpose | 引物名称 Primer name | 引物序列 Primer sequence | 退火温度/℃ Temperature | 延伸时间/s Time |
|---|---|---|---|---|
| miR408 qPCR | dlo-miR408-3p-qF | ATGCACTGCCTCTTCCCTGGC | 60 | 30 |
| dlo-miR408-5p1-qF | ACGGGGAACAGGCAGAGCATG | 60 | 30 | |
| miRNA qRT-PCR | Univer-primer | GATCGCCCTTCTACGTCGTAT | 60 | 30 |
| pre-miR408 qPCR | pre-miR408-qF | AGAGCAGCAAGAGACAAAGACG | 60 | 30 |
| pre-miR408-qR | GAAAGAGGAGAAGAAGGAGCC | 60 | 30 | |
| pri-miR408 qPCR | pri-miR408-qF | GGCTTCACCCATGCACTGC | 60 | 30 |
| pri-miR408-qR | TCACTCACCCAGTAATCAGTTGC | 60 | 30 | |
| 靶基因定量Target gene qPCR | DlLAC12-qF | GCTTCTCCTAGCTTCTACATTGTCC | 60 | 30 |
| DlLAC12-qR | CAAAGTTGTCAACAAAGCCAG | 60 | 30 | |
| 内参基因Reference gene/miRNA | DlFSD-qF | GGTCAGATGGTGAAGCCGTAGAG | 60 | 30 |
| DlFSD-qR | GTCTATGCCACCGATACAACAAACCC | 60 | ||
| dlo-miR156a*-qF | TGCTCACTTCTCCTCTGTCAG | 60 | 30 | |
| pri-miR408 5RACE | pri-miR408-5R1 | CAGGGAAGAGGCAGTGCATGG | 56.7 | 30 |
| pri-miR408-5R2 | GCATCCATGCTCTGCCTGTTC | 54.8 | 45 | |
| pri-miR408 3RACE | pri-miR408-3R1 | ACAGGCAGAGCATGGATGCAACTA | 55 | 45 |
| pri-miR408-3R2 | GGCTTCACCCATGCACTGC | 54 | ||
| pri-miR408 cDNA | pri-miR408-F | TTGTGGAAGGAAACGTGG | 56 | 45 |
| pri-miR408-R | TGAACCTGCATTTCTGTCTACAC | |||
| pro-MIR408 | pro-MIR408-F | CGAGTGAGATGAATCCGAAACAAAG | 65 | 90 |
| pro-MIR408-R | CGAGTGAGATGAATCCGAAACAAAG | |||
| DlLAC12 cDNA | DlLAC12-F | ATGGAGCTTCTCAAAAGCAC | 56 | 120 |
| DlLAC12-R | CACCAGATTTGCCTATATGCTAA |
表1 基因克隆及qRT-PCR所用引物
Table 1 Primers used in gene cloning and qRT-PCR
| 用途 Purpose | 引物名称 Primer name | 引物序列 Primer sequence | 退火温度/℃ Temperature | 延伸时间/s Time |
|---|---|---|---|---|
| miR408 qPCR | dlo-miR408-3p-qF | ATGCACTGCCTCTTCCCTGGC | 60 | 30 |
| dlo-miR408-5p1-qF | ACGGGGAACAGGCAGAGCATG | 60 | 30 | |
| miRNA qRT-PCR | Univer-primer | GATCGCCCTTCTACGTCGTAT | 60 | 30 |
| pre-miR408 qPCR | pre-miR408-qF | AGAGCAGCAAGAGACAAAGACG | 60 | 30 |
| pre-miR408-qR | GAAAGAGGAGAAGAAGGAGCC | 60 | 30 | |
| pri-miR408 qPCR | pri-miR408-qF | GGCTTCACCCATGCACTGC | 60 | 30 |
| pri-miR408-qR | TCACTCACCCAGTAATCAGTTGC | 60 | 30 | |
| 靶基因定量Target gene qPCR | DlLAC12-qF | GCTTCTCCTAGCTTCTACATTGTCC | 60 | 30 |
| DlLAC12-qR | CAAAGTTGTCAACAAAGCCAG | 60 | 30 | |
| 内参基因Reference gene/miRNA | DlFSD-qF | GGTCAGATGGTGAAGCCGTAGAG | 60 | 30 |
| DlFSD-qR | GTCTATGCCACCGATACAACAAACCC | 60 | ||
| dlo-miR156a*-qF | TGCTCACTTCTCCTCTGTCAG | 60 | 30 | |
| pri-miR408 5RACE | pri-miR408-5R1 | CAGGGAAGAGGCAGTGCATGG | 56.7 | 30 |
| pri-miR408-5R2 | GCATCCATGCTCTGCCTGTTC | 54.8 | 45 | |
| pri-miR408 3RACE | pri-miR408-3R1 | ACAGGCAGAGCATGGATGCAACTA | 55 | 45 |
| pri-miR408-3R2 | GGCTTCACCCATGCACTGC | 54 | ||
| pri-miR408 cDNA | pri-miR408-F | TTGTGGAAGGAAACGTGG | 56 | 45 |
| pri-miR408-R | TGAACCTGCATTTCTGTCTACAC | |||
| pro-MIR408 | pro-MIR408-F | CGAGTGAGATGAATCCGAAACAAAG | 65 | 90 |
| pro-MIR408-R | CGAGTGAGATGAATCCGAAACAAAG | |||
| DlLAC12 cDNA | DlLAC12-F | ATGGAGCTTCTCAAAAGCAC | 56 | 120 |
| DlLAC12-R | CACCAGATTTGCCTATATGCTAA |

图1 pri-miR408及其靶标DlLAC12克隆和成熟体序列(蓝色)及前体序列(划线部分) M:Marker;胞嘧啶“C”为转录起始位点。
Fig. 1 Gene cloning of pri-miR408 and DlLAC12,miR408(blue)and pre-miR408 sequence(underlined) M:Marker;“C”is the transcription start site.
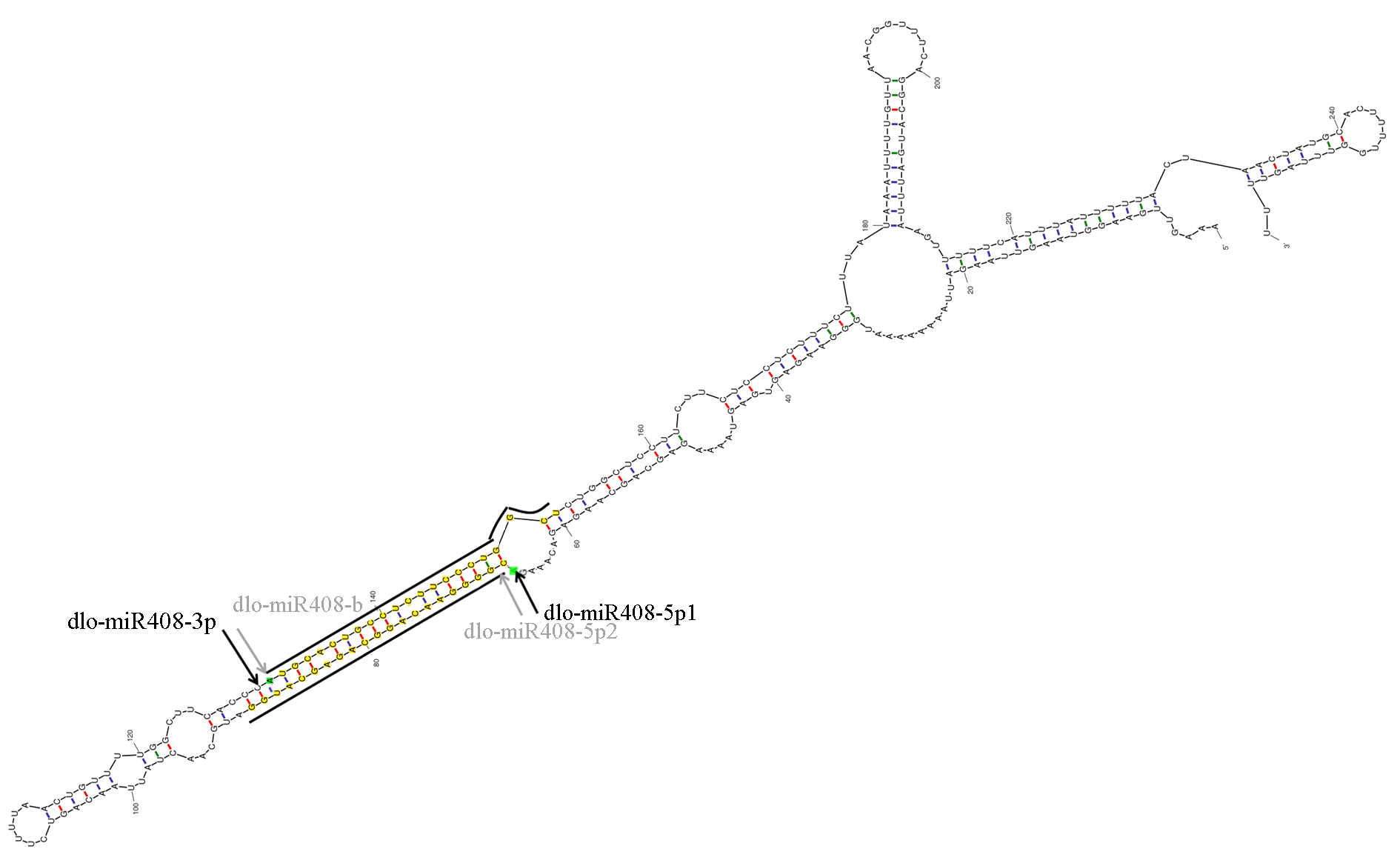
图3 dlo-pre-miR408序列二级结构 箭头代表miRNA 5′端第1个碱基
Fig. 3 The secondary structure of dlo-pre-miR408 sequence stem-loop structure The arrows point to the first base of 5′ of miRNA.

图4 龙眼dlo-miR408(A)、dlo-MIR408(B)与植物多物种聚类分析 32种植物的miR408,Dlo:龙眼Dimocarpus longan;stu:马铃薯Solanum tuberosum;nta:烟草Nicotiana tabacum;dpr:紫花洋地黄Digitalis purpurea;gma:大豆Glycine max;bdi:二穗短柄草Brachypodium distachyon;aly:琴叶拟南芥Arabidopsis lyrata;vvi:葡萄Vitis vinifera;pta:火炬松Pinus taeda;sof:甘蔗Saccharum officinarum;ath:拟南芥Arabidopsis thaliana;osa:水稻Oryza sativa;ppt:小立碗藓Physcomitrella patens;sbi:高粱sorghum bicolor;ahy:花生Arachis hypogaea;ssp:甘蔗Saccharum ssp;mes:木薯Manihot esculenta;mdm:苹果Malus domestica;cpa:番木瓜Carica papaya;ptc:毛果杨Populus trichocarpa;tae:小麦Triticum aestivum;rco:蓖麻Ricinus communis;vun:豇豆Vigna unguiculata;cme:香瓜Cucumis melo;ata:山羊草Aegilops tauschii;zma:玉米Zea mays;csi:甜橙Citrus sinensis;mtr:蒺藜苜蓿Medicago truncatula;cca:刺菜蓟Cynara cardunculus;bra:芜菁Brassica rapa;lja:百脉根Lotus japonicus;hbr:橡胶树Hevea brasiliensis.miRNA前体聚类分析共选取33种植物,Dlo:龙眼Dimocarpus longan;rco:蓖麻Ricinus communis;cpa:番木瓜Carica papaya;hbr:橡胶树Hevea brasiliensis;mes:木薯Manihot esculenta;ptc:毛果杨Populus trichocarpa;vvi,葡萄Vitis vinifera;mtr:蒺藜苜蓿Medicago truncatula;mdm:苹果Malus domestica;aqc:耧斗菜Aquilegia caerulea;stu:马铃薯Solanum tuberosum;cca:刺菜蓟Cynara cardunculus;pta:火炬松Pinus taeda;ppt:小立碗藓Physcomitrella patens;smo:江南卷柏Selaginella moellendorffii;sof:甘蔗Saccharum officinarum;gma,大豆Glycine max;ssp:甘蔗Saccharum ssp;dpr:毛地黄Digitalis purpurea;cme:香瓜Cucumis melo;zma:玉米Zea mays;ata:山羊草Aegilops tauschii;tae,小麦Triticum aestivum;bdi:二穗短柄草Brachypodium distachyon;osa:水稻Oryza sativa;sbi:高粱sorghum bicolor;bra:芜菁Brassica rapa;ath:拟南芥Arabidopsis thaliana;Aly:琴叶拟南芥Arabidopsis lyrata;csi:甜橙Citrus sinensis;vun:豇豆Vigna unguiculata;Lus:亚麻Linum usitatissimum;lja:百脉根Lotus japonicus.
Fig. 4 The phylogenetic tree construction of dlo-miR408(A)and dlo-MIR408(B)nucleotide sequence in multiple plant species
| 顺式作用元件 Cis-acting element | 位点数 Number of site | 正、负链 Positive and negative chain | 矩阵分数 Matrix fraction | 核心序列 Core sequence | 注释 Annotation | |
|---|---|---|---|---|---|---|
| ABRE | 1 | + | 9 | GCAACGTGTC | 参与脱落酸反应性 Participates in abscisic acid reactivity | |
| ARE | 1 | - | 6 | AAACCA | 厌氧诱导 Anaerocabically | |
| Box 4 | 2 | +/- | 6 | ATTAAT | 光响应有关保守DNA模块 Light response related to conserved DNA modules | |
| CAT-box | 2 | -/+ | 6 | GCCACT | 分生组织表达相关 Meristem expression | |
| CGTCA-motif | 1 | + | 5 | CGTCA | 参与MeJA反应 Participate in MeJA responsing | |
| GARE-motif | 1 | - | 7 | TCTGTTG | 赤霉素应答 Gibberellin response | |
| GT1-motif | 1 | + | 6 | GGTTAA | 光响应元件 Light response element | |
| LTR | 2 | +/- | 6 | CCGAAA | 参与低温反应 Participate in low temperature | |
| MYB | 4 | +/-/+/- | 6 | CAACAG、CAACCA、TAACCA | MYB结合位点 MYB binding site | |
| MYB-like sequence | 1 | - | 6 | TAACCA | ||
| MYC | 1 | - | 6 | CATTTG | MYC结合位点MYC binding site | |
| Myb-binding site | 2 | + | 6 | CAACAG | ||
| Myc | 1 | - | 7 | TCTCTTA | ||
| TATC-box | 2 | - | 7 | TATCCCA | 赤霉素反应Gibberellin response | |
| TCCC-motif | 1 | - | 7 | TCTCCCT | 光响应Light response | |
| TGACG-motif | 1 | - | 5 | TGACG | 参与MeJA反应Participate in MeJA response | |
表2 pro-MIR408顺式作用元件分析
Table 2 The cis-acting element analysis of pro-MIR408
| 顺式作用元件 Cis-acting element | 位点数 Number of site | 正、负链 Positive and negative chain | 矩阵分数 Matrix fraction | 核心序列 Core sequence | 注释 Annotation | |
|---|---|---|---|---|---|---|
| ABRE | 1 | + | 9 | GCAACGTGTC | 参与脱落酸反应性 Participates in abscisic acid reactivity | |
| ARE | 1 | - | 6 | AAACCA | 厌氧诱导 Anaerocabically | |
| Box 4 | 2 | +/- | 6 | ATTAAT | 光响应有关保守DNA模块 Light response related to conserved DNA modules | |
| CAT-box | 2 | -/+ | 6 | GCCACT | 分生组织表达相关 Meristem expression | |
| CGTCA-motif | 1 | + | 5 | CGTCA | 参与MeJA反应 Participate in MeJA responsing | |
| GARE-motif | 1 | - | 7 | TCTGTTG | 赤霉素应答 Gibberellin response | |
| GT1-motif | 1 | + | 6 | GGTTAA | 光响应元件 Light response element | |
| LTR | 2 | +/- | 6 | CCGAAA | 参与低温反应 Participate in low temperature | |
| MYB | 4 | +/-/+/- | 6 | CAACAG、CAACCA、TAACCA | MYB结合位点 MYB binding site | |
| MYB-like sequence | 1 | - | 6 | TAACCA | ||
| MYC | 1 | - | 6 | CATTTG | MYC结合位点MYC binding site | |
| Myb-binding site | 2 | + | 6 | CAACAG | ||
| Myc | 1 | - | 7 | TCTCTTA | ||
| TATC-box | 2 | - | 7 | TATCCCA | 赤霉素反应Gibberellin response | |
| TCCC-motif | 1 | - | 7 | TCTCCCT | 光响应Light response | |
| TGACG-motif | 1 | - | 5 | TGACG | 参与MeJA反应Participate in MeJA response | |
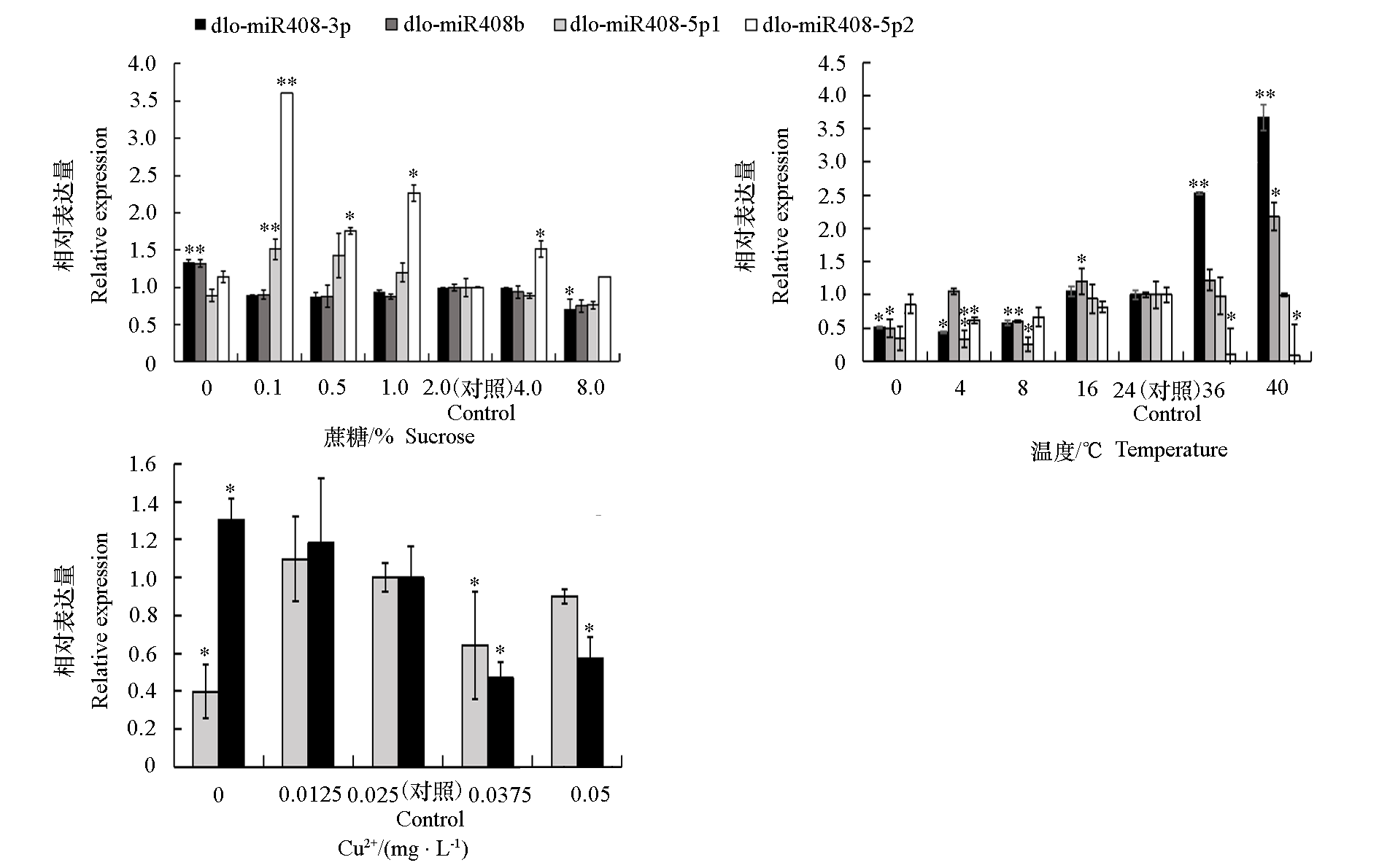
图7 dlo-miR408在不同浓度蔗糖、铜离子和不同温度胁迫下的表达模式 “*”代表与对照相比在0.05水平具有显著性差异,“**”代表在0.01水平下具有显著性差异,每个处理设置3个独立的生物学重复。
Fig. 7 Expression patterns of dlo-miR408 during different concentrations of sucrose,Cu2+ and different temperatures “*”represents significant difference at 0.05 level,“**”represents significant difference at 0.01 level. Every treatment sets up three independent biological replicates.
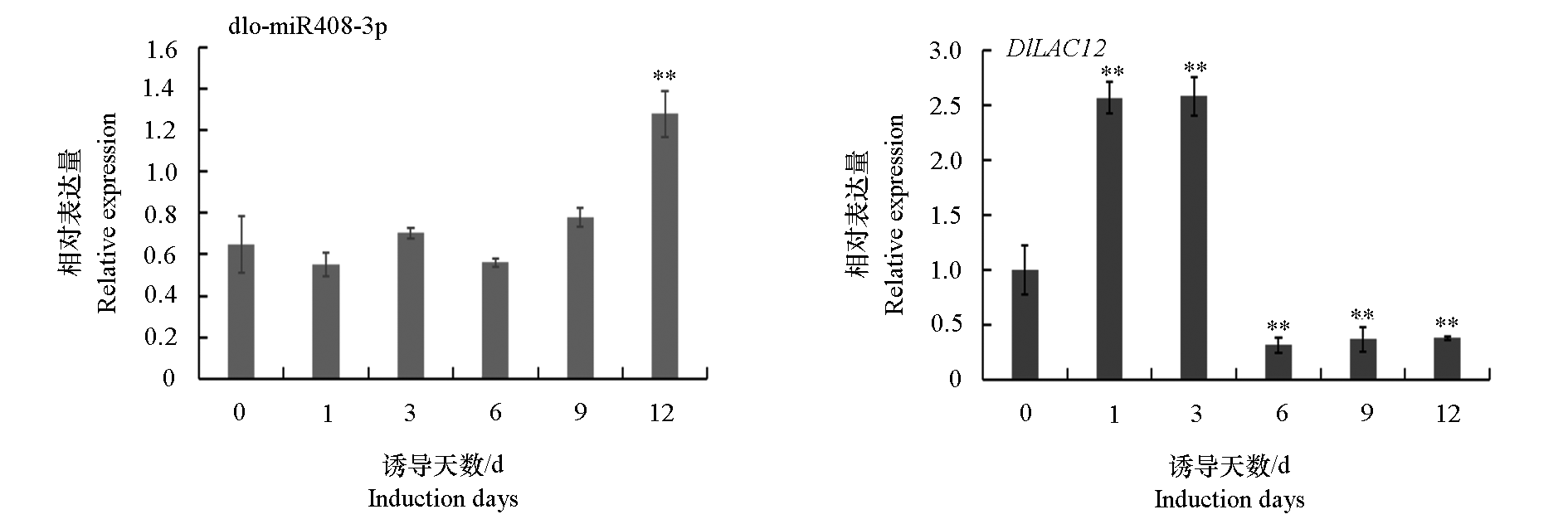
图8 dlo-miR408与DlLAC12在龙眼球形胚不同诱导阶段的表达模式 ** 代表P < 0.01水平下具有极显著性差异。每个处理设置3个独立的生物学重复。
Fig. 8 Different expression patterns of dlo-miR408 and DlLAC12 during different days of GE induction in longan ** represents a very significant difference at P < 0.01 level. Every treatment sets up three independent biological replicates.
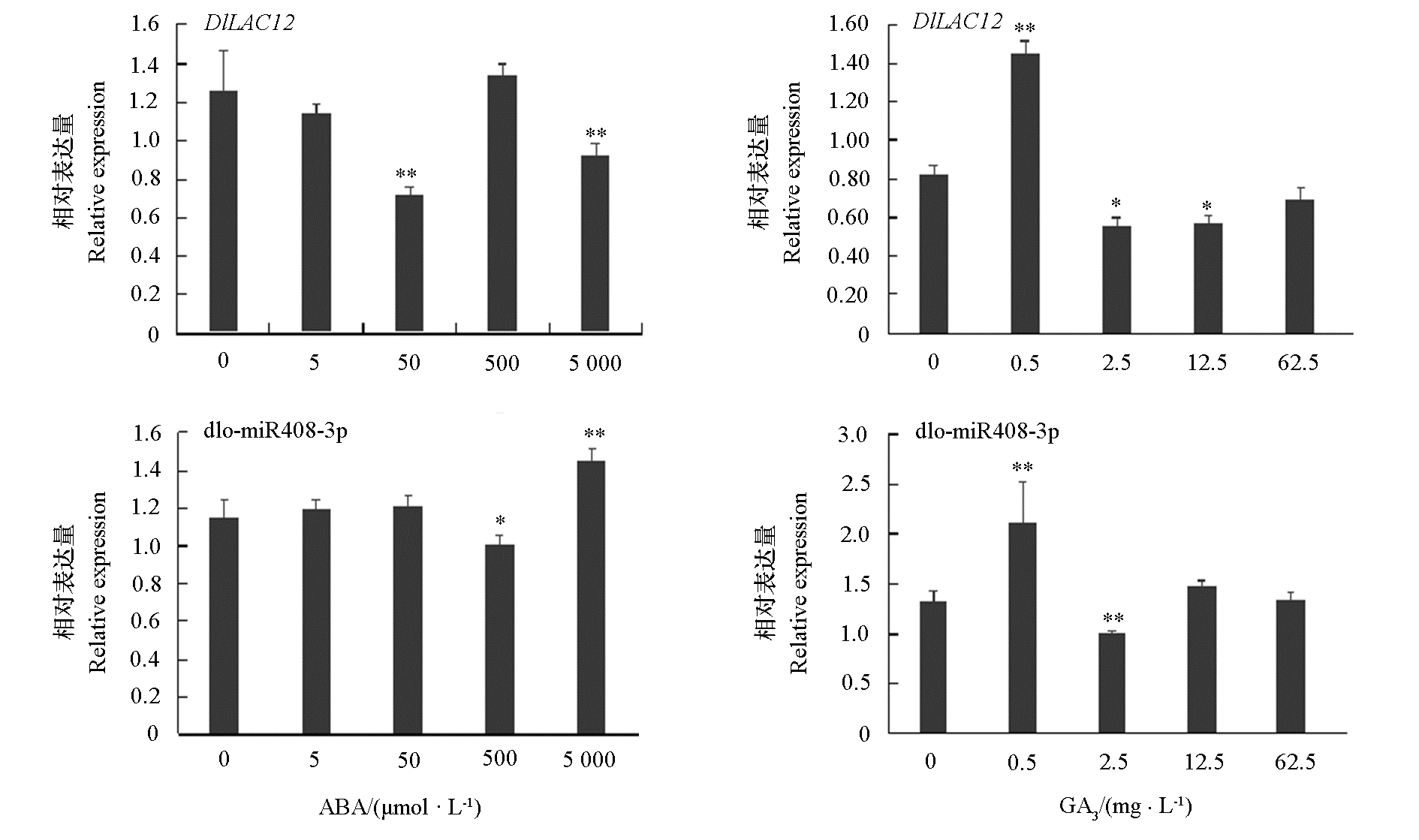
图9 dlo-miR408与靶基因DlLAC12在不同浓度ABA和GA3处理24 h的定量表达分析 * 代表P < 0.05水平下具有显著性差异,** 代表P < 0.01水平下具有极显著性差异。
Fig. 9 The different expression analysis of dlo-miR408-3p and DlLAC12 during different concentration of ABA and GA3 * represents significant difference at P < 0.05. ** represents a very significant difference at P < 0.01 level.
| [1] | Che Jian-mei, Lai Zhong-xiong, Lai Cheng-chun, Guo Zhi-xiong, Liu Hong-zhou, Huang Zhi-hong. 2005. Changes of endogenous phytohormones during the early somatic. Chinese Journal of Tropical, 26 (2):55-61. (in Chinese) |
| 车建美, 赖钟雄, 赖呈纯, 郭志雄, 刘鸿洲, 黄志宏. 2005. 荔枝体细胞胚胎发生早期的3种内源激素含量变化. 热带作物学报, 26 (2):55-61. | |
| [2] |
Chen C J, Liu Q, Zhang Y C, Qu L H, Chen Y Q, Gautheret D. 2011. Genome-wide discovery and analysis of microRNAs and other small RNAs from rice embryogenic callus. RNA Biology, 8 (3):538-547.
doi: 10.4161/rna.8.3.15199 URL |
| [3] | Chen Yao-feng, Wang Li-min, Ding Yun-jiao, Li Chun-lian, Guo Yue-xia, Han De-jun, Ren Hui-li, Guo Dong-wei, Xi Ya-jun. 2004. Effect of copper on dedifferention and high frequency reg enera tion of imm ature embryos in wheat(Triticum aestivum L.). Journal of Northwest Sci Tech University of Agriculture and Forestry, 32 (10):1-4. (in Chinese) |
| 陈耀锋, 王丽敏, 丁云姣, 李春莲, 郭月霞, 韩德俊, 任慧莉, 郭东伟, 奚亚军. 2004. Cu2+对小麦幼胚脱分化和高频再生特性的影响. 西北农林科技大学学报(自然科学版), 32 (10):1-4. | |
| [4] | Guo Xiao-rong, Yang Xin-bing, Wang Huai-qin, Ming Fang-lin, She Xu, Cao Xiao-yan. 2016. Clonging and expression analysisof miR408 precursor sequences from Salvia miltiorrhiza. Plant Science Journal, 34 (3):430-438. (in Chinese) |
| 郭晓荣, 杨新兵, 王怀琴, 明方琳, 佘旭, 曹晓燕. 2016. 丹参miR408基因前体序列的克隆及表达分析. 植物科学学报, 34 (3):430-438. | |
| [5] |
Hajyzadeh M, Turktas M, Khawar K M, Unver T. 2015. miR408 overexpression causes increased drought tolerance in chickpea. Gene, 555 (2):186-193.
doi: 10.1016/j.gene.2014.11.002 pmid: 25445265 |
| [6] | Jiang A L, Guo Z L, Pan J W, Zhuang Y, Zuo D Q, Hao C, Gao Z X, Xin P Y, Chu J F, Zhong S W, Li L. 2021. The PIF1-MIR408-plantacyanin repression cascade regulates light-dependent seed germination. Plant Cell, 22:koab060. |
| [7] | Kong De-yan. 2010. Cloning and primary functional study of drought tolerance relate miRNAs in rice[M. D. Dissertation]. Wuhan: Huazhong Agriculture University. (in Chinese) |
| 孔德艳. 2010. 水稻抗旱相关miRNAs的克隆及其功能的初步研究[硕士论文]. 武汉: 华中农业大学. | |
| [8] | Lai Zhong-xiong, Chen Chun-ling. 2002. Changes of endogenous phytohormones in the process of somatic embryogenesis in longan (Dimocarpus longan Lour.). Chinese Journal of Tropical Crops, 23 (2):41-46. (in Chinese) |
| 赖钟雄, 陈春玲. 2002. 龙眼体细胞胚胎发生过程中的内源激素变化. 热带作物学报, 23(2):41-46. | |
| [9] | Lai Zhong-xiong, Chen Zhen-guang. 1997. Somatic embryogenesis of high frequency from longan embryogenic calli. Journal of Fujian Agricultural University, 26 (3):271-276. (in Chinese) |
| 赖钟雄, 陈振光. 1997. 龙眼胚性愈伤组织的高频率体细胞胚胎发生. 福建农业大学学报, 26 (3):271-276. | |
| [10] | Lai Zhong-xiong, Pan Zhen-liang, Chen Zhen-guang. 1997. Establishment and maintenance of longan embryogenic cell lines. Journal of Fujian Agricultural University, 26 (2):160-167. (in Chinese) |
| 赖钟雄, 潘振良, 陈振光. 1997. 龙眼胚性细胞系的建立与保持. 福建农业大学学报, 26 (2):160-167. | |
| [11] | Lauressergues D, Couzigou J M, Clemente H S, Martinez Y, Dunand C, Bécard G, Combier J P. 2015. Primary transcripts of microRNAs encode,regulatory peptides. Nature, 7545 (520):90-93. |
| [12] | Li Li-chao, Sun Hua-yu, Yang Yi-hong, Zhao Han-sheng, Wang Si-ning, Gao Zhi-min. 2017. Cloning and expression analysis of miR398 and miR408 in Phyllostachysedulis. Journal of Tropical and Subtropical Botany, 25 (3):241-249. (in Chinese) |
| 李利超, 孙化雨, 杨意宏, 赵韩生, 王思宁, 高志民. 2017. 毛竹 miR398和miR408的克隆及其表达分析. 热带亚热带植物学报, 25 (3):241-249. | |
| [13] |
Lin Y L, Lai Z X. 2010 Reference gene selection for qPCR analysis during somatic embryogenesis in longan tree. Plant Science, 178 (4):359-365.
doi: 10.1016/j.plantsci.2010.02.005 URL |
| [14] | Lin Y L, Lin L X, Lai R L, Liu W H, Chen Y K, Zhang Z H, Xu XuHan, Lai Z X. 2015a. MicroRNA390-directed TAS3 cleavage leads to the production of tasiRNA-ARF3/ 4 during somatic embryogenesis in Dimocarpus longan Lour. Frontiers in Plant Science, 6:1119. |
| [15] |
Lin Y L, Lai Z X. 2013. Evaluation of suitable reference genes for normalization of microRNA expression by real-time reverse transcription PCR analysis during longan somatic embryogenesis. Plant Physiology and Biochemistry, 66:20-25.
doi: 10.1016/j.plaphy.2013.02.002 pmid: 23454294 |
| [16] | Lin Y L, Tian Q L, Lin L X, Lai R L, Yang M M, Zhang D M, Chen Y K, Zhang Z H, Lai Z X. 2015b. Endogenous target mimics down-regulate miR160 mediation of ARF10,-16,and -17 cleavage during somatic embryogenesis in Dimocarpus longan Lour. Frontiers in Plant Science, 5 (6):956. |
| [17] | Lin Yu-ling. 2011. Studies on cloning,Expression and regulation of SOD gene family during somatic embryogenesis in Dimocarpus longan Lour. [Ph. D. Dissertation]. Fuzhou:Fujian Agriculture and Forestry University. (in Chinese) |
| 林玉玲. 2011. 龙眼体胚发生过程中SOD基因家族的克隆及表达调控研究[博士论文]. 福州: 福建农林大学. | |
| [18] | Liu Hua-ying. 2003. Studies on the characters in cytohistology and physiology and bioehemistry of somatic embryogenesis in Citrus. [Ph. D. Dissertation]. Changsha:Hunan Agriculture University. (in Chinese) |
| 刘华英. 2003. 柑橘体细胞胚发生的细胞学及生理生化特性研究[博士论文]. 长沙: 湖南农业大学. | |
| [19] | Liu Wei-hua, Ni Shan-shan, Lin Zheng-chun, Lin Yu-ling, Lai Zhong-xiong. 2017. Evolution and molecular characteristics of the miR408 family in plants. Chin J Appl Environ Biol, 23 (6):1042-1051. (in Chinese) |
| 刘炜婳, 倪珊珊, 林争春, 林玉玲, 赖钟雄. 2017. 植物miR408家族的进化与分子特性. 应用与环境生物学报, 23 (6):1042-1051. | |
| [20] | Liu Yan-ying. 2020. Study on the response mechanism of miR408 to low temperature stress in banana tissue culture seedings[M. D. Dissertation]. Fuzhou: Fujian Agriculture University. (in Chinese) |
| 刘彦英. 2020. miR408在香蕉组培苗中低温胁迫下响应机制的研究[硕士论文]. 福州: 福建农林大学. | |
| [21] | Liu Yanying, Ni Shanshan, Xiang Leilei, Chen Yukun, Lai Zhongxiong. 2020. Genome-wide identification of the laccase gene family and its expression analysis under low temperature stress in Musa accuminata. Acta Horticulturae Sinica, 47 (5):837-852. (in Chinese) |
| 刘彦英, 倪珊珊, 项蕾蕾, 陈裕坤, 赖钟雄. 2020. 香蕉漆酶基因家族鉴定及低温胁迫下的表达分析. 园艺学报, 47 (5):837-852. | |
| [22] | Ma C, Burd S, Lers A. 2015. miR408 is involved in abiotic stress responses in Arabidopsis. Plant Journal, 84:169-187. |
| [23] | Ma Sheng-yun. 2012. Expression pattern and function of miR408 in seed development of rice(Oryza sativa)[M. D. Dissertation]. Hangzhou: Zhejiang University. (in Chinese) |
| 马圣运. 2012. Os-miR408的表达模式及其在水稻种子发育中的功能[硕士论文]. 杭州: 浙江大学. | |
| [24] | Maunoury N, Vaucheret H. 2011. AGO1 and AGO 2 act redundantly in miR408-mediated plantacyanin regulation. PLoS ONE, 12 (6):e28729. |
| [25] |
Peng D L, Chen X G, Yin Y P, Lu K L, Yang W B, Tang Y H, Wang Z L. 2014. Lodging resistance of winter wheat(Triticum aestivum L.):Lignin accumulation and its related enzymes activitiesdue to the application of paclobutrazol or gibberellin acid. Field Crops Research, 157:1-7.
doi: 10.1016/j.fcr.2013.11.015 URL |
| [26] |
Ren L G, Tang G L. 2012. Identification of sucrose-responsive microRNAs reveals sucrose-regulated copper accumulations in an SPL7-dependent and independent manner in Arabidopsis thaliana. Plant Science, 187:59-68.
doi: 10.1016/j.plantsci.2012.01.014 URL |
| [27] |
Song Z Q, Zhang L F, Wang Y L, Li H X, Zhao H J, Zhang H Y. 2018. Constitutive expression of miR408 improves biomass and seed yield in Arabidopsis. Frontiers in Plant Science, 8:2114.
doi: 10.3389/fpls.2017.02114 URL |
| [28] |
Soulanki M, Sinha A, Shuklal I. 2019. The miR408 expression in scutellum derived somatic embryos of Oryza sativa L. ssp. indica varieties:media and regenerating embryos. Plant Cell,Tissue and Organ Culture, 138:53-66.
doi: 10.1007/s11240-019-01602-w URL |
| [29] |
Sun M Z, Yang J K, Cai X X, Shen Y, Cui N, Zhu Y M, Jia B W, Sun X L. 2018. The opposite roles of OsmiR408 in cold and drought stress responses in Oryza sativa. Mol Breeding, 38:120.
doi: 10.1007/s11032-018-0877-z URL |
| [30] |
Sunkar R, Zhu J K. 2004. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell, 16:2001-2019.
doi: 10.1105/tpc.104.022830 URL |
| [31] | Townsley B T, Sinha N R, Kang J L. 2013. KNOX1 genes regulate lignin deposition and composition in monocots and dicots. Frontiers in plant Science, 121 (4):1-11. |
| [32] |
Trindade I, Capita C, Dalmay T, Fevereiro M P,dos Santos D M. 2010. miR398 and miR408 are up-regulated in response to water deficit in Medicago truncatula. Planta, 231:705-716.
doi: 10.1007/s00425-009-1078-0 pmid: 20012085 |
| [33] | Xu X P, Chen X H, Chen Y, Zhang Q L, Su L Y, Chen X, Chen Y K, Zhang Z H, Lin Y L, Lai Z X. 2020. Genome-wide identification of miRNAs and their targets during early somatic embryogenesis in Dimocarpus longan Lour. Scientific Report, 1 (10):4626. |
| [34] |
Yadav S K, Kumar V V S, Verma K, Yadav P, Saroha A, Wankhede D P, Chaudhary B, Chinnusamy V. 2020. Genome-wide identification and characterization of ABA receptor PYL gene family in rice. BMC Genomics, 21:676.
doi: 10.1186/s12864-020-07083-y URL |
| [35] | Yang X F, Zhao X L, Dai Z Y, Ma F L, Miao X X, Shi Z Y. 2021. OsmiR396/growth regulating factor modulate rice grain size through direct regulation of embryo-specific miR408. Plant Physiology,1-15. |
| [36] |
Zhang H Y, Li L. 2013. SQUAMOSA promoter binding protein-like7 regulated microRNA408 is required for vegetative development in Arabidopsis. Plant Journal, 74:98-109.
doi: 10.1111/tpj.12107 URL |
| [37] | Zhang H Y, Zhao X, Li J G, Cai H Q, Deng X W, Li L. 2014. MicroRNA408 is critical for the HY5-SPL7 gene network that mediates the coordinated response to light and copperPlant Cell, 26:4933-4953. |
| [38] | Zhang J H, Zhang S G, Han S Y, Li X X, Tong Z K, Qi L W. 2013. Deciphering small noncoding RNAs during the transition from dormant embryo to germinated embryo in larches(Larix leptolepis). PLoS ONE, 8 (12):81452. |
| [39] |
Zhang J P, Yu Y, Feng Y Z, Zhou Y F, Zhang F, Yang Y W, Lei M Q, Zhang Y C, Chen Y Q. 2017. MiR408 regulates grain yield and photosynthesis via a phytocyanin protein. Plant Physiology, 175:1175-1185.
doi: 10.1104/pp.17.01169 URL |
| [40] | Zhang Jin-ping, Li Quan-feng, Zhang Yu-chan, Yu Yang, Feng Yan-zhao, Chen Yue-qin. 2019. The application of miR408 gene and UCL in cultivating high-yield rice. China:CN105112422B. 2019-11-08. (in Chinese) |
| 张金平, 李权峰, 张玉婵, 于洋, 冯彦钊, 陈月琴. 2019. 基因miR408和UCL在培育高产水稻中的应用. 中国:CN105112422B. 2019-11-08. | |
| [41] |
Zhao X Y, Hong P, Wu J Y, Chen X B, Ye X G, Pan Y Y, Wang J, Zhang X S. 2016. The tae-miR408-mediated control of TaTOC1 genes transcription is required for the regulation of heading time in wheat. Plant Physiology, 170:1578-1594.
doi: 10.1104/pp.15.01216 URL |
| [1] | 邓朝军, 许奇志, 蒋际谋, 胡文舜, 郑少泉, 陈秀萍, 姜 帆, 许家辉, 苏文炳, 张雅玲, 黄敬峰. 浓香型龙眼新品种‘醇香’[J]. 园艺学报, 2022, 49(S2): 75-76. |
| [2] | 邓朝军, 陈秀萍, 许奇志, 蒋际谋, 郑少泉, 胡文舜, 姜 帆, 许家辉, 苏文炳, 张雅玲, 黄敬峰. 浓香型龙眼新品种‘福香’[J]. 园艺学报, 2022, 49(S2): 77-78. |
| [3] | 贾鑫, 曾臻, 陈月, 冯慧, 吕英民, 赵世伟. 月季‘月月粉’RcDREB2A的克隆与表达分析[J]. 园艺学报, 2022, 49(9): 1945-1956. |
| [4] | 马维峰, 李艳梅, 马宗桓, 陈佰鸿, 毛娟. 苹果POD家族基因的鉴定与MdPOD15的功能分析[J]. 园艺学报, 2022, 49(6): 1181-1199. |
| [5] | 肖学宸, 刘梦雨, 蒋梦琦, 陈燕, 薛晓东, 周承哲, 吴兴健, 吴君楠, 郭寅生, 叶开温, 赖钟雄, 林玉玲. 龙眼褪黑素合成途径SNAT、ASMT和COMT家族基因鉴定及表达分析[J]. 园艺学报, 2022, 49(5): 1031-1046. |
| [6] | 刘梦雨, 蒋梦琦, 陈燕, 张舒婷, 薛晓东, 肖学宸, 赖钟雄, 林玉玲. 龙眼GDSL酯酶/脂肪酶基因的全基因组鉴定及表达分析[J]. 园艺学报, 2022, 49(3): 597-612. |
| [7] | 周至铭, 杨佳宝, 张程, 曾令露, 孟晚秋, 孙黎. 向日葵LACS家族鉴定及响应非生物胁迫表达分析[J]. 园艺学报, 2022, 49(2): 352-364. |
| [8] | 申序, 陈晓慧, 张婧, 陈荣珠, 徐小萍, 李晓斐, 蒋梦琦, 刘蒲东, 倪珊珊, 林玉玲, 赖钟雄. 龙眼染色质重塑因子Snf2基因家族的进化动力学研究及在体胚发生早期的表达[J]. 园艺学报, 2022, 49(1): 41-61. |
| [9] | 谢思艺, 周承哲, 朱晨, 詹冬梅, 陈兰, 吴祖春, 赖钟雄, 郭玉琼. 茶树CsTIFY家族全基因组鉴定及非生物胁迫和激素处理中主要基因表达分析[J]. 园艺学报, 2022, 49(1): 100-116. |
| [10] | 梁志乐, 汪宽鸿, 杨静, 祝彪, 朱祝军. 硫代葡萄糖苷在十字花科植物应对非生物胁迫中的作用[J]. 园艺学报, 2022, 49(1): 200-220. |
| [11] | 张春渝, 许小琼, 徐小萍, 赵鹏程, 申序, MunirNigarish, 张梓浩, 林玉玲, 陈振光, 赖钟雄. 龙眼SKP1-like家族成员鉴定及体胚发生早期表达分析[J]. 园艺学报, 2021, 48(9): 1665-1679. |
| [12] | 杨为海, 曾利珍, 肖秋生, 石胜友. 饥饿胁迫下龙眼落果与果皮和离区糖、ABA及相关基因表达的变化[J]. 园艺学报, 2021, 48(8): 1457-1469. |
| [13] | 杨天宸, 陈晓童, 吕可, 张荻. 百子莲脱水素基因ApSK3对逆境与激素信号的应答模式与调控机制[J]. 园艺学报, 2021, 48(8): 1565-1578. |
| [14] | 马俊杰, 宋丽娜, 李乐, 马晓春, 靳磊, 徐伟荣. 山葡萄VaCBL6参与非生物胁迫和ABA途径的响应[J]. 园艺学报, 2021, 48(6): 1079-1093. |
| [15] | 苏立遥, 王培育, 蒋梦琦, 黄倏祺, 薛晓东, 刘梦雨, 肖学宸, 赖春旺, 张梓浩, 陈裕坤, 赖钟雄, 林玉玲. 龙眼pri-miR319a编码短肽活性的研究[J]. 园艺学报, 2021, 48(5): 908-920. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司