
Acta Horticulturae Sinica ›› 2025, Vol. 52 ›› Issue (8): 1987-2020.doi: 10.16420/j.issn.0513-353x.2024-0969
• Reviews • Previous Articles Next Articles
TAN Xiaoyu1, NIU Junpeng2,3, WANG Quanzhi1, ZHANG Li2, ZHU Lijuan2, WANG Guodong3, ZHENG Heyun4, YU Zhifang2, DUAN Yuquan5, JIANG Li2, SUN Xiuxiu6, YANG Ying7, LUO Weiqi6, LI Xuehui2, GUAN Le2, ZHAO Yanling1, LI Guoxiao1, YIN Congfei1, GE Cheng1, MA Min2, JIA Luting2, ZHANG Xu2, ZHAO Yaoyao5,*( ), GENG Xinli4,*(
), GENG Xinli4,*( ), WANG Libin2,8,*(
), WANG Libin2,8,*( ), and ZHANG Shaoling2,*(
), and ZHANG Shaoling2,*( )
)
Received:2025-06-13
Revised:2025-07-15
Online:2025-08-19
Published:2025-08-19
Contact:
ZHAO Yaoyao, GENG Xinli, WANG Libin, and ZHANG Shaoling
TAN Xiaoyu, NIU Junpeng, WANG Quanzhi, ZHANG Li, ZHU Lijuan, WANG Guodong, ZHENG Heyun, YU Zhifang, DUAN Yuquan, JIANG Li, SUN Xiuxiu, YANG Ying, LUO Weiqi, LI Xuehui, GUAN Le, ZHAO Yanling, LI Guoxiao, YIN Congfei, GE Cheng, MA Min, JIA Luting, ZHANG Xu, ZHAO Yaoyao, GENG Xinli, WANG Libin, and ZHANG Shaoling. Recent Advances in JAs-Induced Improvement of Postharvest Chilling Resistance in Horticultural Crops[J]. Acta Horticulturae Sinica, 2025, 52(8): 1987-2020.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.ahs.ac.cn/EN/10.16420/j.issn.0513-353x.2024-0969
| 园艺作物 Horticultural crop | 冷害症状 Chilling injury symptoms | 临界冻害温度/℃ Critical chilling temperature |
|---|---|---|
| 果树Fruit tree | ||
| 苹果(亚热带季风气候区) Apple(Subtropical monsoon climate region) | 内部变褐,褐芯,脆化,软烫伤 Internal browning,brown core,soggy breakdown,soft scald | 2 ~ 3 |
| 凤梨释迦Atemoya | 表皮变黑,不成熟,果肉变色Skin darkening,failure to ripen,pulp discoloration | 4 |
| 油梨Avocado | 果肉灰褐色褪色Grayish-brown discoloration of flesh | 4.5 ~ 13 |
| 木橘Bael | 表皮出现褐色斑点Brown spots on skin | 3 |
| 香蕉(绿色或成熟) Banana(green or ripe) | 成熟时颜色暗淡Dull color when ripened | 11.5 ~ 13 |
| 面包果Breadfruit | 非正常成熟,暗褐色褪色Abnormal ripening,dull brown discoloration | 7 ~ 12 |
| 蔓越莓Cranberry | 橡胶质地,红褐色果肉Rubbery texture,red flesh | 2 |
| 番石榴Guava fruit | 果肉损伤,腐烂Pulp injury,decay | 4.5 |
| 西柚Grapefruit | 烫伤痕迹,点状腐蚀,水渍Scald,pitting,watery breakdown | 10 |
| 柠檬Lemon | 点状腐蚀,膜质染色,红色斑点Pitting,membranous staining,red blotch | 11 ~ 13 |
| 酸橙Lime | 点状腐蚀,随着时间推移变成棕褐色Pitting,turning tan with time | 7 ~ 9 |
| 荔枝Lychee | 表皮变褐Skin browning | 3 |
| 杧果Mango | 表皮呈灰鳞片状褪色,成熟不均匀 Grayish scald-like discoloration of skin,uneven ripening | 10 ~ 13 |
| 山竹Mangosteen | 皮层硬化褐变Hardening and browning of the cortex | 4 ~ 8 |
| 新鲜橄榄Olive,fresh | 内部变褐Internal browning | 7 |
| 橘Orange | 点状腐蚀,棕色污渍Pitting,brown stain | 3 |
| 木瓜Papaya | 不成熟,变味,腐烂Pitting,failure to ripen,off-flavor,decay | 7 |
| 西番莲Passion fruit | 表皮褪色成暗红色,味道变淡,腐烂 Dark red discoloration on skin,loss of flavor,decay | 10 |
| 菠萝Pineapple | 成熟后呈暗绿色Dull green when ripened | 7 ~ 10 |
| 石榴Pomegranate | 点状腐烂,内外部褐变Pitting,external and internal browning | 4.5 |
| 红毛丹Rambutan | 外果皮变黑Darkening of exocarp | 10 |
| 树番茄Tamarillo | 表面点状腐蚀,褪色Surface pitting,discoloration | 3 ~ 4 |
| 瓜类Melons and gourds | ||
| 哈密瓜Cantaloup | 点状腐蚀,表面腐化Pitting,surface decay | 2 ~ 5 |
| 网纹瓜Crenshaw and Persian | 红褐色褪色,麻点,表面腐烂,不成熟 Reddish-tan discoloration,pitting,surface decay,failure to ripen | 7 ~ 10 |
| 蜜瓜Honeydew | 红褐色褪色,麻点,表面腐烂,不成熟 Reddish-tan discoloration,pitting,surface decay,failure to ripen | 7 ~ 10 |
| 白兰瓜Casaba | 红褐色褪色,麻点,表面腐烂,不成熟 Reddish-tan discoloration,pitting,surface decay,failure to ripen | 7 ~ 10 |
| 西瓜Watermelon | 点状腐蚀,异味Pitting,objectionable flavor | 4.5 |
| 蔬菜(果菜)Vegetables(fruits and vegetables) | ||
| 佛手瓜Choyote | 暗褐色褪色,点状腐蚀,果肉变黑Dull brown discoloration,pitting,flesh darkening | 5 ~ 10 |
| 黄瓜Cucumber | 点状腐蚀,水渍状斑点,腐烂Pitting,water-soaked spots,decay | 7 |
| 茄子Eggplant | 表皮有烫伤痕迹,黑斑病,种子变黑Surface scald,alternaria rot,blackening of seeds | 7 |
| 豆薯Jicama | 表面腐烂,褪色Surface decay,discoloration | 13 ~ 18 |
| 秋葵Okra pod | 褪色,水浸区域,点状腐蚀,腐烂Discoloration,water-soaked areas,pitting,decay | 7 |
| 南瓜和硬壳南瓜 Pumpkin and hard-shell squash | 腐烂,特别是黑斑病Decay,especially alternaria rot | 10 |
| 利马豆Phaseolus lunatus | 锈褐色斑块、斑点或区域Rusty brown specks,spots,or areas | 0 ~ 2 |
| 菜豆Phaseolus vulgaris | 点状腐蚀和褐斑Pitting and russeting | 7 |
| 辣椒(甜)Pepper(sweet) | 豆荚和花萼上的片状腐烂,黑斑病,种子变黑 Sheet pitting,alternaria rot on pods and calyxes,darkening of seed | 7 |
| 西红柿(成熟期)Tomato(ripe) | 浸水,软化,腐烂Water-soaking and softening,decay | 7 ~ 10 |
| 西红柿(绿熟期)Tomato(mature green) | 成熟时色泽差,黑斑病Poor color when ripe,alternaria rot | 13 |
| 蔬菜(叶菜)Vegetables(leafy vegetables) | ||
| 石刁柏Asparagus | 暗淡,灰绿色,尖端软弱无力Dull,gray-green,and limp tips | 0 ~ 2 |
| 蕹菜Water convolvulus | 茎叶变黑Darkening of leaves and stems | 10 |
Table 1 Chilling injury symptoms of different fruits and vegetables with their critical chilling temperatures
| 园艺作物 Horticultural crop | 冷害症状 Chilling injury symptoms | 临界冻害温度/℃ Critical chilling temperature |
|---|---|---|
| 果树Fruit tree | ||
| 苹果(亚热带季风气候区) Apple(Subtropical monsoon climate region) | 内部变褐,褐芯,脆化,软烫伤 Internal browning,brown core,soggy breakdown,soft scald | 2 ~ 3 |
| 凤梨释迦Atemoya | 表皮变黑,不成熟,果肉变色Skin darkening,failure to ripen,pulp discoloration | 4 |
| 油梨Avocado | 果肉灰褐色褪色Grayish-brown discoloration of flesh | 4.5 ~ 13 |
| 木橘Bael | 表皮出现褐色斑点Brown spots on skin | 3 |
| 香蕉(绿色或成熟) Banana(green or ripe) | 成熟时颜色暗淡Dull color when ripened | 11.5 ~ 13 |
| 面包果Breadfruit | 非正常成熟,暗褐色褪色Abnormal ripening,dull brown discoloration | 7 ~ 12 |
| 蔓越莓Cranberry | 橡胶质地,红褐色果肉Rubbery texture,red flesh | 2 |
| 番石榴Guava fruit | 果肉损伤,腐烂Pulp injury,decay | 4.5 |
| 西柚Grapefruit | 烫伤痕迹,点状腐蚀,水渍Scald,pitting,watery breakdown | 10 |
| 柠檬Lemon | 点状腐蚀,膜质染色,红色斑点Pitting,membranous staining,red blotch | 11 ~ 13 |
| 酸橙Lime | 点状腐蚀,随着时间推移变成棕褐色Pitting,turning tan with time | 7 ~ 9 |
| 荔枝Lychee | 表皮变褐Skin browning | 3 |
| 杧果Mango | 表皮呈灰鳞片状褪色,成熟不均匀 Grayish scald-like discoloration of skin,uneven ripening | 10 ~ 13 |
| 山竹Mangosteen | 皮层硬化褐变Hardening and browning of the cortex | 4 ~ 8 |
| 新鲜橄榄Olive,fresh | 内部变褐Internal browning | 7 |
| 橘Orange | 点状腐蚀,棕色污渍Pitting,brown stain | 3 |
| 木瓜Papaya | 不成熟,变味,腐烂Pitting,failure to ripen,off-flavor,decay | 7 |
| 西番莲Passion fruit | 表皮褪色成暗红色,味道变淡,腐烂 Dark red discoloration on skin,loss of flavor,decay | 10 |
| 菠萝Pineapple | 成熟后呈暗绿色Dull green when ripened | 7 ~ 10 |
| 石榴Pomegranate | 点状腐烂,内外部褐变Pitting,external and internal browning | 4.5 |
| 红毛丹Rambutan | 外果皮变黑Darkening of exocarp | 10 |
| 树番茄Tamarillo | 表面点状腐蚀,褪色Surface pitting,discoloration | 3 ~ 4 |
| 瓜类Melons and gourds | ||
| 哈密瓜Cantaloup | 点状腐蚀,表面腐化Pitting,surface decay | 2 ~ 5 |
| 网纹瓜Crenshaw and Persian | 红褐色褪色,麻点,表面腐烂,不成熟 Reddish-tan discoloration,pitting,surface decay,failure to ripen | 7 ~ 10 |
| 蜜瓜Honeydew | 红褐色褪色,麻点,表面腐烂,不成熟 Reddish-tan discoloration,pitting,surface decay,failure to ripen | 7 ~ 10 |
| 白兰瓜Casaba | 红褐色褪色,麻点,表面腐烂,不成熟 Reddish-tan discoloration,pitting,surface decay,failure to ripen | 7 ~ 10 |
| 西瓜Watermelon | 点状腐蚀,异味Pitting,objectionable flavor | 4.5 |
| 蔬菜(果菜)Vegetables(fruits and vegetables) | ||
| 佛手瓜Choyote | 暗褐色褪色,点状腐蚀,果肉变黑Dull brown discoloration,pitting,flesh darkening | 5 ~ 10 |
| 黄瓜Cucumber | 点状腐蚀,水渍状斑点,腐烂Pitting,water-soaked spots,decay | 7 |
| 茄子Eggplant | 表皮有烫伤痕迹,黑斑病,种子变黑Surface scald,alternaria rot,blackening of seeds | 7 |
| 豆薯Jicama | 表面腐烂,褪色Surface decay,discoloration | 13 ~ 18 |
| 秋葵Okra pod | 褪色,水浸区域,点状腐蚀,腐烂Discoloration,water-soaked areas,pitting,decay | 7 |
| 南瓜和硬壳南瓜 Pumpkin and hard-shell squash | 腐烂,特别是黑斑病Decay,especially alternaria rot | 10 |
| 利马豆Phaseolus lunatus | 锈褐色斑块、斑点或区域Rusty brown specks,spots,or areas | 0 ~ 2 |
| 菜豆Phaseolus vulgaris | 点状腐蚀和褐斑Pitting and russeting | 7 |
| 辣椒(甜)Pepper(sweet) | 豆荚和花萼上的片状腐烂,黑斑病,种子变黑 Sheet pitting,alternaria rot on pods and calyxes,darkening of seed | 7 |
| 西红柿(成熟期)Tomato(ripe) | 浸水,软化,腐烂Water-soaking and softening,decay | 7 ~ 10 |
| 西红柿(绿熟期)Tomato(mature green) | 成熟时色泽差,黑斑病Poor color when ripe,alternaria rot | 13 |
| 蔬菜(叶菜)Vegetables(leafy vegetables) | ||
| 石刁柏Asparagus | 暗淡,灰绿色,尖端软弱无力Dull,gray-green,and limp tips | 0 ~ 2 |
| 蕹菜Water convolvulus | 茎叶变黑Darkening of leaves and stems | 10 |
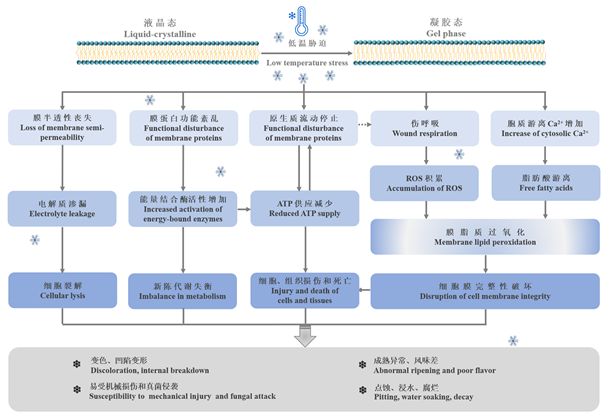
Fig. 1 Physio-biochemical responses of horticultural crops to chilling temperature Wang,1989;Paull,1990;Theocharis et al.,2012. Cryogenic temperature triggers a series of the complicated physio-biochemical alternations in plant cell,leading to the occurrence of chilling injury symptom. Firstly,cell membrane was changed from liquid crystal phase to gelatinous phase,resulting in the loss of membrane semi-permeability,disfunction of membrane proteins,decrement of protoplasm fluidity,and so on. In accompany with the occurrence of electrolyte leakage,energy reduction,and oxidative stress,cell membrane damage is exacerbated. If the chilling-exposure continues,cell membrane would be irreversibly damaged,causing chilling injury symptom
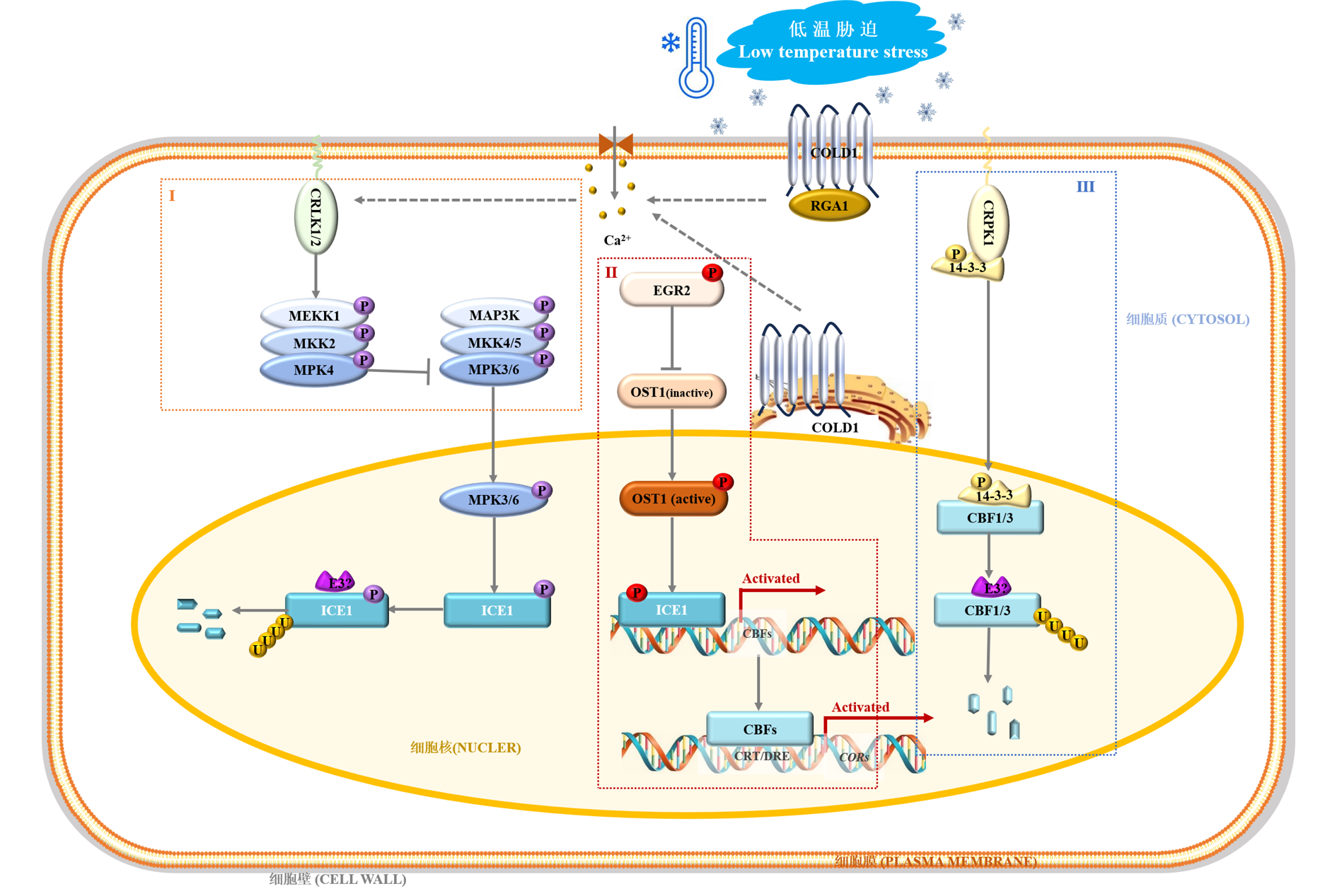
Fig. 2 CBF-dependent signaling pathway in plants Li et al.,2017a;Liu et al.,2017b;Ding et al.,2019. The ICE1-CBF-COR transcriptional cascade is of great importance for the CBF-dependent signaling pathway. During chilling stress,the membrane protein complex COLD1-RGA1 senses environmental signals to induce Ca2+ influx. After perception of Ca2+ signals,CRLK and EGR2-OST1-ICE1 signaling pathways are activated to enhance the stability and transcriptional activity of ICE1,resulting in the upregulated expression levels of CBFs. On the other hand,the CRPK1 signal transduction pathway,which negatively regulates the stability of CBFs,is also initiated to avoid the cellular over-response to low temperature. After translation,CBFs could bind to cis-acting element(CRT/DRE)in the promoters of the downstream CORs genes and then initiate their transcription. CBF:C-repeat binding factors;COLD1:Chilling tolerance divergence 1;COR:Cold responsive gene;CRLK:Calcium/calmodulin-regulated receptor-like kinase;CRPK1:Cold-responsive protein kinase 1;EGR2:Clade-E growth-regulating 2;MAP3K:Mitogen-activated protein kinase kinase kinase;MEKK:MAPK/ERK Kinase Kinase;MKK:MAPK Kinase;MPK:Mitogen-activated protein kinase;ICE1:Inducer of CBF expression 1;OST1:Open stomata 1; P:Phosphorylation;RGA1:Rice G-protein α subunit 1
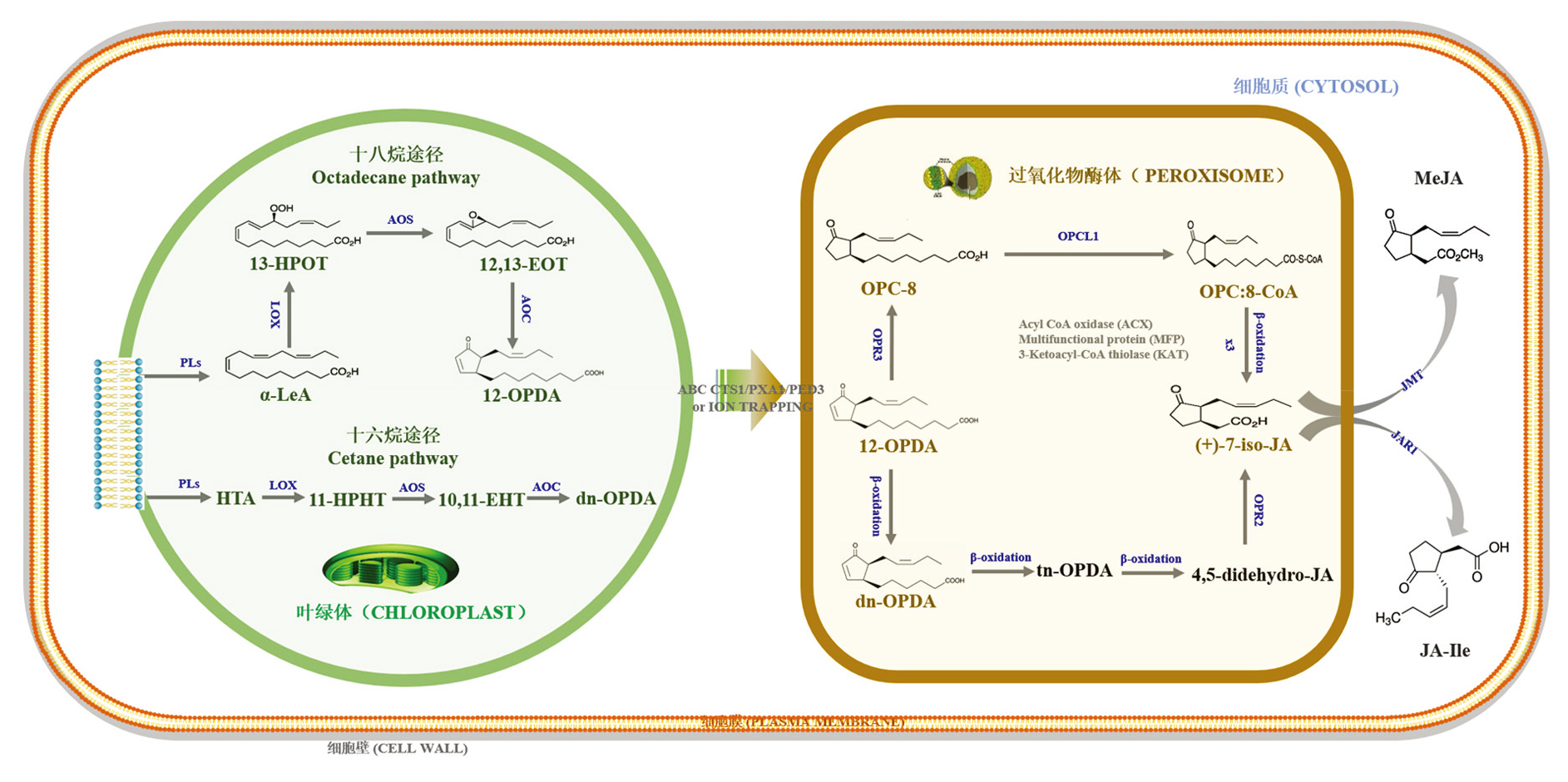
Fig. 3 Biosynthetic pathway of JAs in plants Sharma & Laxmi,2016;Ali & Baek,2020;Wan & Xin,2022. JAs is derived from α-LeA and HTA via oxidation,cyclization,reduction,β-oxidation,modification and activation. Upon release from chloroplast membrane,α-LeA and HTA are catalyzed by LOX,AOS and AOC into 12-OPDA and dn-OPDA,which are then transported into peroxisome by ABC transporter or ionic barrier. In peroxisome,12-OPDA and dn-OPDA are converted into (+)-7-iso-JA via the actions of several enzymes,including OPR2/3,OPCL1,ACX,MFP and KAT. After transportation into the cytoplasm,(+)-7-iso-JA could be catalyzed by JAR1 and JMT to form JA-Ile and MeJA,respectively. ACX:Acyl-CoA oxidase;AOC:Alle-neoxide cyclase;AOS:Allene oxide synthase;4,5-didehydro-JA:4,5-Didehydro-jasmonic acid;dn-OPDA:Dinor OPDA;10,11-EHT:10,11(S)-epoxy- hexadeca(tri)enoic acid;12,13-EOT:12,13-(S)-epoxy-octadecatrienoic acid;11-HPHT:11-Hydroperoxyhexadecatrienoic acid;13-HPOT:13-Hydroperoxyoctadecatrienoic acid;HTA:Hexadecatrienoic acid;(+)-7-iso-JA:(+)-7-iso-Jasmonic acid;JA-Ile:Jasmonoyl-isoleucine;JAR1:Jasomonate resistant 1;JMT:Jasmonic acid carboxyl methyltransferase;KAT:3-Ketoacyl-CoA thiolase;α-LeA:α-Linolenic acid;LOX:Lipoxygenases;OPC-8:3-Oxo-2-((Z)-pent-2-en-1-yl)cyclopentane-1-octanoic acid;MeJA:Methyl jasmonate;MFP:Multifunctional protein;OPC:8-CoA:3-Oxo-2-((Z)-pent-2-en-1-yl)cyclopentane-1-octanoyl-CoA;OPCL1:OPC-8 coenzyme A ligase1;12-OPDA:12-Oxo-phytodienoic acid;OPR:OPDA reductase;PLs:Phospholipases;tn-OPDA:Tetranor-OPDA
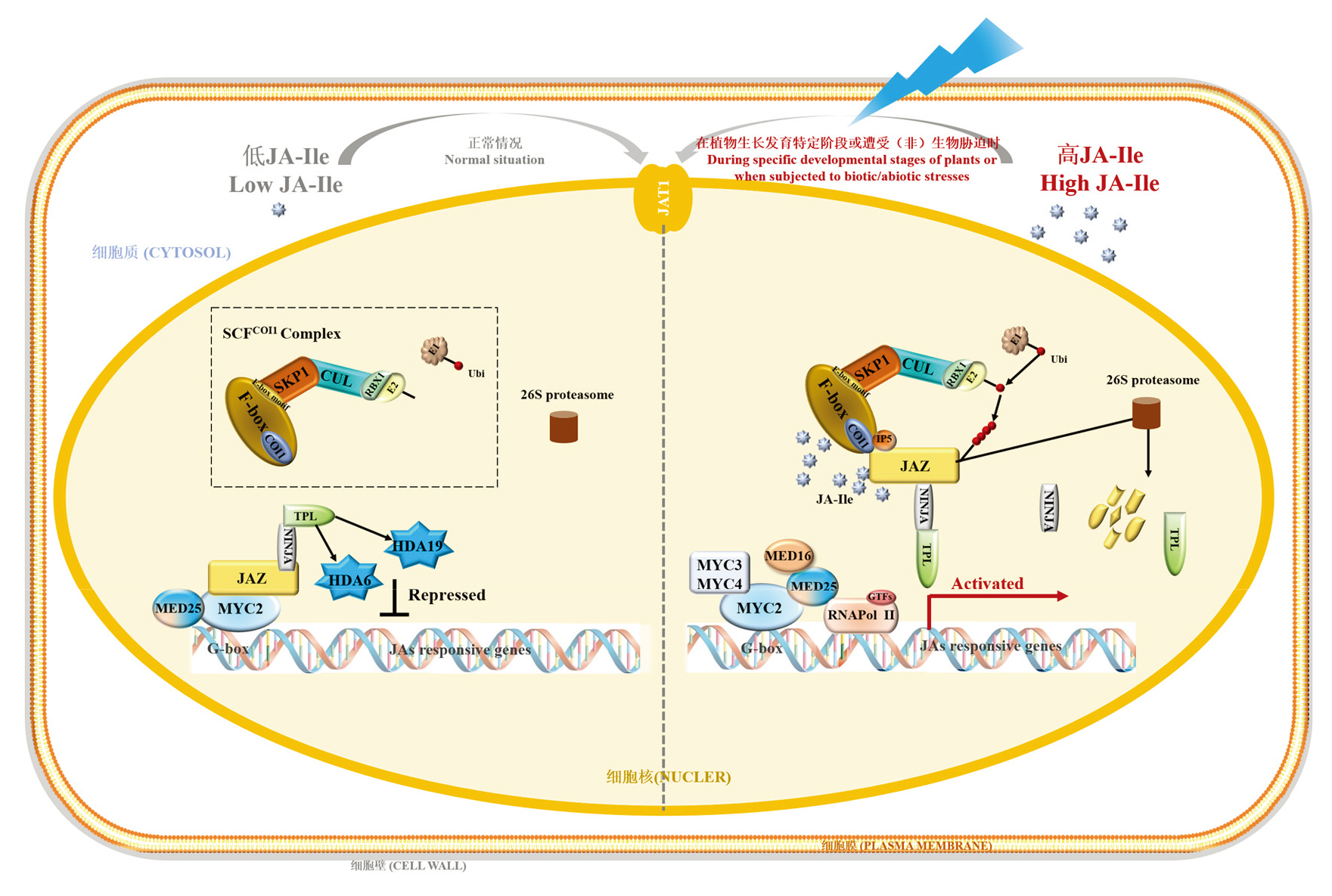
Fig. 4 JAs signal transduction process in plants Sharma & Laxmi,2016;Ali & Baek,2020;Li et al.,2022. JA-Ile is transported from cytosol into the nucleus with the aid of JAT1 to initiate its signal transduction process. When JA-Ile is low,the repressor protein JAZs recruits NINJA,TPL,and HDA6/19 to form a closed transcriptional inhibition complex to inhibit MYC2 transcriptional activity. At the specific developmental stages or under(a)biotic stress,the rapid accumulation of JA-Ile in the nucleus promotes the interaction between JAZs and COI1 in the E3 ubiquitin ligase complex SCFCOI1,leading to the ubiquitin-mediated degradation of JAZs by IP5 and 26S proteasome. Then,MYC2 is released from JAZs. Then,MYC2 forms homodimers or heterodimers with its homologs MYC3 and MYC4,which bind to the G-box element. Subsequently,MED25 recruits General Transcription Factors(GTFs)and RNA polymerase II to the promoter to initiate transcription. Additionally,MED16 enhances the stability of MED25 through interaction with it,which is crucial for activating the expression of JAs-responsive genes. COI1:Coronatine insensitive 1;CUL:Cullin;E1,E2:Ubiquitin-conjugating enzymes;GTF:General transcription factor;HDA6,HDA19:Histone deacetylase 6,19;JA:Jasmonic acid;JA-Ile:Jasmonyl isoleucine;JAT1:Jasmonic acid transfer protein 1;JAZ:Jasmonate ZIM domain;MED16,25:Mediator complex subunit 16;MYC:Myelocytomatosis transcription factor;NINJA:Novel interactor of JAZ;RBX1:Ring box 1;RNA Pol II:RNA polymerase II;SCF:Skp1-cullin-F-box e3 ubiquitin ligase complex;SKP1:S-phase kinase-associated protein 1;TPL:Topless;Ubi:Ubiquitin
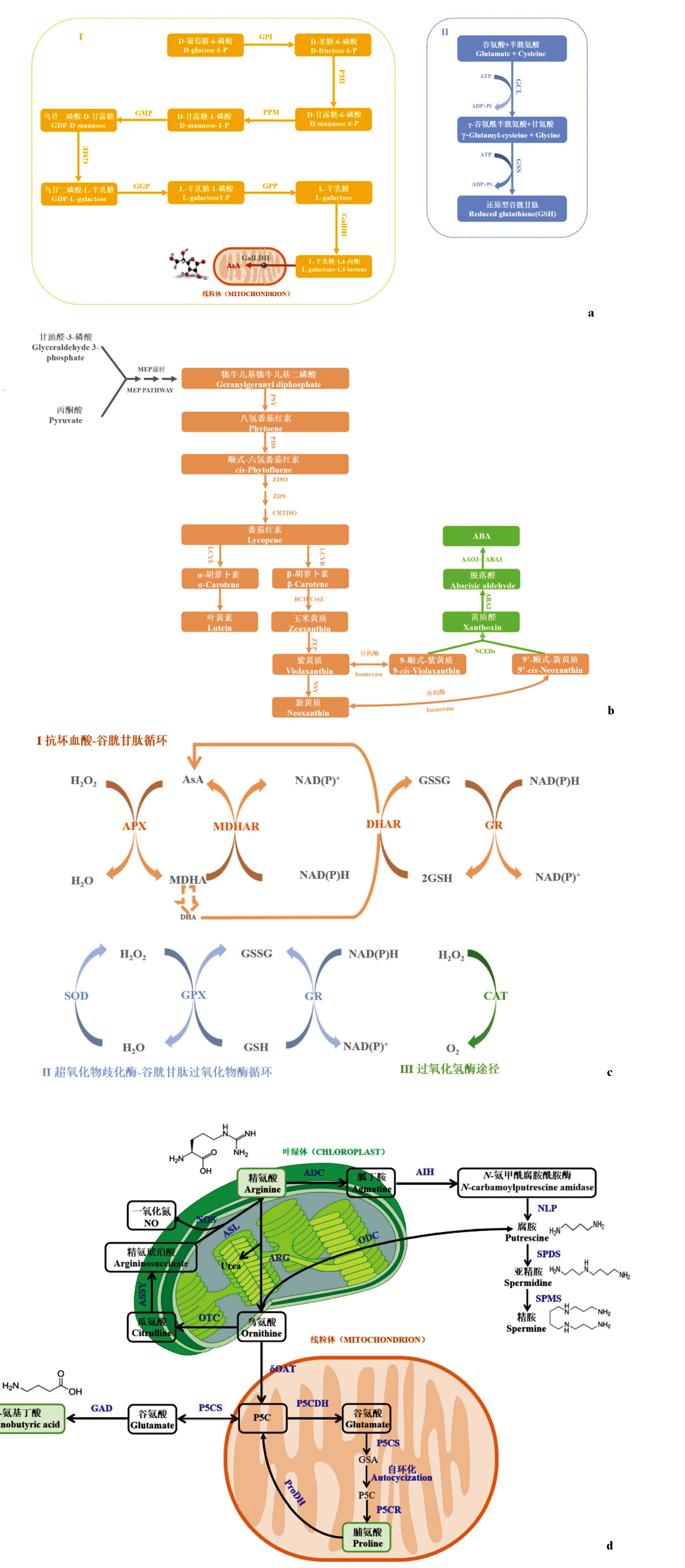
Fig. 5 Synthesis and metabolic pathways of some substances in plants a:The biosynthetic pathways of AsA and GSH in plant(Forman et al.,2009;Ishikawa et al.,2018).(I)D-mannose/L-galactose pathway(also known as Smirnoff Wheeler pathway)is the most important pathway for AsA biosynthesis in plant. D-glucose-6-phosphate in cytosol is converted into L-galactono-1,4-lactone by GPI,PMI,PMM,GMP,GME,GDP-GGP,GPP,and GalDH;after transportation into mitochondria,L-galactono-1,4-lactone is oxidized into AsA with the aid of GalLDH.(II)GSH is derived from glutamic acid through the actions of glutamate-cysteine ligase and glutathione synthase. b:The biosynthetic pathways of carotenoids and abscisic acid in plant(Nurbekova et al.,2021;Sun et al.,2022;Wu et al.,2022b). The biosynthesis of carotenoids and abscisic acid in plant begins with MEP pathway. Geraniol pyrophosphate,which is produced via MEP pathway,is catalyzed by PSY,PDS,ZISO,ZDS and CRTISO to produce lycopene. The latter is then cyclized by LCYE and LCYB to form β-carotene and α-carotene. β-carotene could further be converted into zeaxanthin,violaxanthin,neoxanthin,9-cis-violaxanthin and 9’-cis-neoxanthin with the aid of various enzymes,including BCH/CrtZ,ZEP,NSY and uncharacterized isomerase. Moreover,9-cis-violaxanthin and 9’-cis-neoxanthine,which are derived from the carotenoid-biosynthetic pathway,could be acted as the substrates for ABA formation through the catalytic actions of NCED,ABA2 and AAO3. c:Reactive oxygen species scavenging pathways in plant(Hasanuzzaman et al.,2022).(Ⅰ)Ascorbate-glutathion cycle;(Ⅱ)superoxide dismutase-glutathion peroxidase cycle;(Ⅲ)the catalase pathway. d:Arginine metabolic pathway in plant(Cao et al.,2012;Zhang et al.,2012;Winter et al.,2015). Putrescine in plant is produced from arginine via ADC-AIH-NLP and ARG-ODC pathways. After formation,putrescine would further be converted by SPDS and SPMS into spermidine and spermidine. Ornithine,as an metabolic intermediate during putrescine formation,could be acted as the substrate for arginine regeneration,with the aid of OTC,ASSY and ASL,to maintain its level in plant cell;additionally,ornithine might also be catalyzed by δOAT,P5CS,GAD,P5CDH and P5CR,producing γ-aminobutyric acid or proline. AAO3:Abscisic-aldehyde oxidase;ABA2:Xanthoxin dehydrogenase;ADC:Arginine decarboxylase;ADP:Adenosine diphosphate;AIH:Agmatine iminohydrolase;APX:Ascorbate peroxidase;ARG:Arginase;AsA:Ascorbic acid;ASL:Argininosuccinate lyase;ASSY:Argininosuccinate synthase;ATP:Adenosine triphosphate;BCH/CrtZ:β-Carotenehydroxylase;CRTISO:Carotene isomerase;DHA:Dehydroascorbate;DHAR:Dehydroascorbate reductase;GAD:Glutamate decarboxylase;GalDH:L -galactose dehydrogenase;GalLDH:L -galactono-1,4-lactone dehydrogenase;GCL:Glutamate cysteine ligase;GGP:GDP- L -galactose phosphorylase;GME:GDP-mannose-3’,5’-epimerase;GMP:GDP-mannose pyrophosphorylase;GPI:Glucose-6-phosphate isomerase;GPP:L-galactose-1-phosphate phosphatase;GPX: Glutathione peroxidase;GR:Glutathione reductase;GSA:Glutamic-y-semialdehyde;GSH:Glutathione;GSS:Glutathione synthase;GSSG:oxidized glutathione;H2O2:Hydrogen peroxide;LCYB:Lycopeneβ-cyclase;LCYE:Lycopene ε-cyclase;MDHAR:Monodehydroascorbate reductase;MEP:Methylerythritol phosphate;NCED:9-cis-epoxycarotenoid dioxygenase;NLP:N-carbamoylputrescine amidase;NOS:NO synthase;NSY:Neoxanthinsynthase;δOAT:Ornithine-δ-aminotransferase;ODC:Ornithine decarboxylase;OTC:Ornithine transcarbamylase;PDS:Phytoene desaturase;Pi:Inorganic phosphate;PMI:Mannose-6-phosphate isomerase;PPM:Phosphomanno mutase;ProDH:Proline dehydrogenase;PSY:Phytoene synthase;P5C:Pyrroline-5-carboxylate;P5CDH:Pyrroline-5-carboxylate dehydrogenase;P5CR:Pyrroline-5-carboxylate reductase;P5CS:Pyrroline-5-carboxylate synthetase;SOD:Superoxide dismutase;SPDS:Spermidine synthase;SPMS:Spermine synthase;ZDS:ζ-Carotene desaturase;ZEP:Zeaxanthinepoxidase;ZISO:ζ-Carotene isomera

Fig. 6 Mechanism on the JAs-induced improvement of chilling resistance in horticultural crops Bolt et al.,2017;Sun et al.,2019;An et al.,2021,2022;Song et al.,2022. Low temperature induces JAs biosynthesis. Afterwards,JAs could directly or indirectly activate the CBF-(in)dependent signal transduction pathway with the aid of several signaling transduction elements,triggering a series of the complicated physio-biochemical alternation(including increment of antioxidant biosynthesis,improvement of antioxidant enzyme activity,inhibition of lipid metabolism,enhancement of arginine metabolism,promotion of energy metabolism,and activation of other plant hormone signal transduction pathways). The abovementioned responses could maintain the integrity,fluidity,and functionality(semi-permeability)of cell membrane,thereby improving the resistance of horticultural crops to chilling stress. ABA:Abscisic acid;ABI4:ABA insensitive 4;ABI5-like:ABA insensitive 5-like;ACX:Acyl-CoA oxidase;AOC:Alle-neoxide cyclase;AOS:Allene oxide synthase;BBX37:B-box protein BBX37;CBF:C-repeat binding factors;COI1:Coronatine insensitive 1;COR:Cold responsive genes;CUL:Cullin;4,5-didehydro-JA:4,5-Didehydro- jasmonic acid;dn-OPDA:Dinor OPDA;E1,E2:Ubiquitin-conjugating enzymes;10,11-EHT:10,11(S)-epoxy-hexadeca (tri)enoic acid;12,13-EOT:12,13-(S)-epoxy-octadecatrienoic acid;ERF:Ethylene responsive factor;ETH:Ethylene;11-HPHT:11-Hydroperoxyhexadecatrienoic acid;13-HPOT:13-Hydroperoxyoctadecatrienoic acid;HTA:Hexadecatrienoic acid;ICE1:Inducer of CBF expression 1;(+)-7-iso-JA:(+)-7-iso-Jasmonic acid;JA-Ile:Jasmonoyl-isoleucine;JAR1:Jasomonate resistant 1;JAT1:Jasmonic acid transfer protein 1;JAZ:Jasmonate ZIM domain;JMT:Jasmonic acid carboxyl methyltransferase;KAT:3-Ketoacyl-CoA thiolase;α-LeA:α-Linolenic acid;LOX:Lipoxygenases;MeJA:Methyl jasmonate;MFP:Multifunctional protein;MYC:Myelocytomatosis transcription factor;NINJA:Novel interactor of JAZ;OPC-8:3-Oxo-2-((Z)-pent-2-en-1-yl)cyclopentane-1-octanoic acid;OPC:8-CoA:3-Oxo-2-((Z)-pent-2-en-1-yl)cyclopentane-1-octanoyl-CoA;OPCL1:OPC-8 coenzyme A ligase1;12-OPDA:12-Oxo-phytodienoic acid;OPR:OPDA reductase;PLs:Phospholipases;RBX1:Ring box 1;RNA Pol II:RNA polymerase II;SKP1:S-phase kinase-associated protein 1;tn-OPDA:Tetranor-OPDA;TPL:Topless;Ubi:Ubiquitin
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [125] |
doi: 10.1007/s00299-013-1414-5 pmid: 23508256 |
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
doi: 10.1016/j.foodchem.2016.01.140 pmid: 26920301 |
| [130] |
|
| [131] |
|
| [8] |
doi: 10.1007/s00425-014-2088-0 pmid: 24903359 |
| [9] |
|
| [10] |
doi: 10.1007/978-981-13-1244-1_1 pmid: 30288701 |
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
doi: S1360-1385(18)30086-4 pmid: 29735429 |
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
doi: 10.1007/s00425-012-1641-y pmid: 22526498 |
| [144] |
|
| [145] |
|
| [22] |
|
| [23] |
|
| [24] |
doi: 10.16420/j.issn.0513-353x.2023-0564 |
|
陈明, 张洁茹, 杨航云, 王印宝, 郑致远, 曾教科, 陈金印, 付永琦, 向妙莲. 2024. 茉莉酸甲酯诱导采后脐橙抗青霉病的机制研究. 园艺学报, 51 (9):2183-2194.
|
|
| [25] |
|
| [26] |
doi: 10.1111/jipb.13087 |
| [27] |
doi: 10.1038/nchembio.2540 pmid: 29291349 |
| [28] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
doi: 10.1007/s00018-015-2089-6 pmid: 26598281 |
| [39] |
|
| [40] |
doi: 10.1105/tpc.110.080788 pmid: 21335373 |
| [41] |
doi: 10.1016/j.pbi.2009.07.013 pmid: 19716757 |
| [42] |
doi: 10.1016/j.mam.2008.08.006 pmid: 18796312 |
| [43] |
|
| [44] |
doi: S0308-8146(17)31637-0 pmid: 29287423 |
| [45] |
doi: 10.1042/bse0580083 pmid: 26374889 |
| [46] |
|
| [47] |
doi: 10.1007/s11103-016-0480-9 pmid: 27086135 |
| [48] |
|
| [49] |
doi: 10.1042/EBC20190085 pmid: 32602544 |
| [50] |
doi: 10.4238/2012.September.10.5 pmid: 23079969 |
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
doi: 10.1105/tpc.113.117796 pmid: 24415770 |
| [57] |
|
| [58] |
doi: 10.1146/annurev-arplant-042817-040047 pmid: 29539269 |
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
doi: 10.1016/j.tplants.2023.03.001 pmid: 37055243 |
| [63] |
|
| [64] |
|
| [155] |
|
| [156] |
|
| [157] |
|
| [158] |
|
| [159] |
|
| [160] |
|
| [161] |
|
| [162] |
|
| [163] |
doi: 10.3389/fpls.2015.00534 pmid: 26284079 |
| [164] |
|
| [165] |
|
| [166] |
|
| [167] |
|
| [168] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
doi: 10.1002/jsfa.5973 pmid: 23208649 |
| [69] |
doi: 10.1016/j.foodchem.2014.03.103 pmid: 24837925 |
| [70] |
|
| [169] |
|
| [170] |
|
| [171] |
|
| [172] |
|
| [173] |
|
| [174] |
doi: 10.1016/j.foodchem.2011.02.011 pmid: 25214120 |
| [175] |
doi: 10.1093/plphys/kiad362 pmid: 37392433 |
| [176] |
|
| [177] |
|
| [178] |
|
| [179] |
doi: 10.1038/hortres.2015.36 pmid: 26504578 |
| [180] |
|
| [181] |
doi: 10.1007/s13197-019-04232-4 pmid: 32431326 |
| [182] |
doi: 10.1016/j.molp.2014.11.001 pmid: 25578269 |
| [183] |
|
| [184] |
|
| [185] |
|
| [186] |
|
| [187] |
|
| [188] |
|
| [189] |
|
| [190] |
|
| [191] |
|
| [192] |
|
| [193] |
|
| [71] |
doi: 10.1016/j.ejmech.2013.09.054 pmid: 24141200 |
| [72] |
|
| [73] |
doi: 10.1105/tpc.111.090100 pmid: 21926335 |
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
doi: 10.1034/j.1600-0854.2003.00086.x pmid: 12656989 |
| [78] |
|
| [194] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
doi: 10.1016/j.foodchem.2013.06.132 pmid: 24001814 |
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
doi: 10.1126/science.8197457 pmid: 8197457 |
| [102] |
doi: 10.1080/10408398.2023.2249097 pmid: 37615648 |
| [103] |
|
| [104] |
doi: 10.1111/nph.17063 pmid: 33131086 |
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
doi: 10.1111/pbi.14276 pmid: 38193234 |
| [110] |
|
| [111] |
|
| [112] |
doi: 10.1038/s41477-018-0254-2 pmid: 30250280 |
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
doi: 10.1007/s00299-020-02614-z pmid: 33034676 |
| [122] |
|
| [123] |
|
| [124] |
doi: 10.1016/s0014-5793(97)01558-5 pmid: 9468303 |
| [1] | BI Qingrui, CUI Dongsheng, MA Xinyuan, XUE Yuran, ZHANG Shikui, FAN Guoquan, NIU Yingying. Genetic Diversity and Genetic Relationship of Local Pear Cultivars in Xinjiang [J]. Acta Horticulturae Sinica, 2025, 52(3): 561-574. |
| [2] | XU Tong, WANG Yue, WU Lina, ZHANG Hang, YIN Lilai, XU Keyu, ZHENG Xiaolin. Effects of Melatonin Treatment on Fruit Quality and Anthocyanin Metabolism of Postharvest‘Taoxingli’Plum [J]. Acta Horticulturae Sinica, 2025, 52(2): 395-405. |
| [3] | LI Yachen, ZHENG Yanmei, SONG Wenpei, LI Dawei, LIANG Hong, ZHANG Xianzhi. Research Progress on the Role of Hydrogen-Rich Water in Plant Growth and Development and Stress Response [J]. Acta Horticulturae Sinica, 2024, 51(7): 1489-1500. |
| [4] | ZHAO Tianrong, LING Jiangang, REN Xiliang. Principal Component Analysis and Genetic Relationship Research on Cultivars of Schlumbergera truncata Based on Morphological Characteristics for DUS Testing [J]. Acta Horticulturae Sinica, 2024, 51(6): 1321-1331. |
| [5] | HUANG Peng, DING Jie, LIU Chunyan, LI Hongying, LI Xinyu, LIU Yaowen, QIN Wen. Comprehensive Evaluation of the Edible Coating on Postharvest Quality of Blueberry [J]. Acta Horticulturae Sinica, 2024, 51(6): 1361-1376. |
| [6] | WU Xiangqi, SUN Li, YU Zheping, YU Qinpei, LIANG Senmiao, ZHENG Xiliang, QI Xingjiang, ZHANG Shuwen. Functional Study of MrSPL4 Gene in Response to Drought and Low Temperature Stress in Chinese Bayberry [J]. Acta Horticulturae Sinica, 2024, 51(5): 927-938. |
| [7] | SUN Quan, HE Zhengchen, YE Junli, WEI Ranran, YIN Yingzi, CHAI Lijun, XIE Zongzhou, XU Qiang, XU Juan, GUO Wenwu, CHENG Yunjiang, DENG Xiuxin. Storage with Climacteric Fruits Improves Color and Quality of Citrus Fruit [J]. Acta Horticulturae Sinica, 2024, 51(3): 601-615. |
| [8] | HE Bin, XU Qinchao, JI Xiaomei, WANG Xiaoling, ZHANG Wendong, CHENG Yunjiang, ZENG Yunliu. Progress of Application on Controlled Atmosphere Preservation Technology in Kiwifruit Storage and Preservation [J]. Acta Horticulturae Sinica, 2023, 50(9): 1916-1928. |
| [9] | DING Jie, LIU Chunyan, HUANG Peng, LI Hongying, CHEN Liwei, PU Xiaoyan, LIU Yaowen, QIN Wen. Recent Advances in Postharvest Technologies to Extend the Shelf Life of Blueberries [J]. Acta Horticulturae Sinica, 2023, 50(9): 1944-1958. |
| [10] | DENG Chengfeng, LI Suping, ZHANG Ruixuan, HAN Leng, LI Yong. Control Effect of Lysobacter enzymogenes LE16 on Rot Disease in Post-harvest Citrus Fruit Caused by Penicillium digitatum and P. italicum [J]. Acta Horticulturae Sinica, 2023, 50(7): 1574-1586. |
| [11] | ZHOU Cheng, FANG Yi, ZHOU Jinyang, HUANG Qihao, PAN Yongjian, SHI Qianqian, NI Huixian, YANG Zhenfeng, SONG Chunbo. The Relationship Between Membrane Lipid Metabolism and Chilling Injury of Postharvest Peach Fruit Induced by Low Temperature [J]. Acta Horticulturae Sinica, 2023, 50(6): 1305-1317. |
| [12] | ZOU Yunqian, LUO Qujuan, ZHANG Jin, XU Rangwei, CHENG Yunjiang. Coating Containing Shellac,Rosin Significantly Improves Commercial Value of Satsuma Mandarins and Lane Late Navel Orange During Shelf Life [J]. Acta Horticulturae Sinica, 2023, 50(4): 853-863. |
| [13] | REN Fei, LU Miaomiao, LIU Jixiang, CHEN Xinli, LIU Daofeng, SUI Shunzhao, MA Jing. Expression and Adversity Resistance Analysis of a Late Embryogenesis Abundant Protein Gene CpLEA from Chimonanthus praecox [J]. Acta Horticulturae Sinica, 2023, 50(2): 359-370. |
| [14] | HE Yafang, BAO Huifang, WANG Ning, ZHAN Faqiang, ZHANG Xuejun, SHI Yingwu, YANG Rong, HOU Xinqiang, LONG Xuanqi. Screening of Antagonistic Bacteria Against Fusarium spp. Causing Melon Fruit Rot and the Antagonistic Properties [J]. Acta Horticulturae Sinica, 2023, 50(10): 2257-2270. |
| [15] | YUAN Xin, XU Yunhe, ZHANG Yupei, SHAN Nan, CHEN Chuying, WAN Chunpeng, KAI Wenbin, ZHAI Xiawan, CHEN Jinyin, GAN Zengyu. Studies on AcAREB1 Regulating the Expression of AcGH3.1 During Postharvest Ripening of Kiwifruit [J]. Acta Horticulturae Sinica, 2023, 50(1): 53-64. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2012 Acta Horticulturae Sinica 京ICP备10030308号-2 国际联网备案号 11010802023439
Tel: 010-82109523 E-Mail: yuanyixuebao@126.com
Support by: Beijing Magtech Co.Ltd