
园艺学报 ›› 2021, Vol. 48 ›› Issue (8): 1565-1578.doi: 10.16420/j.issn.0513-353x.2020-0660
收稿日期:2021-02-09
修回日期:2021-06-03
出版日期:2021-08-25
发布日期:2021-09-06
通讯作者:
张荻
E-mail:zhangdi2013@sjtu.edu.cn
基金资助:
YANG Tianchen, CHEN Xiaotong, LÜ Ke, ZHANG Di( )
)
Received:2021-02-09
Revised:2021-06-03
Online:2021-08-25
Published:2021-09-06
Contact:
ZHANG Di
E-mail:zhangdi2013@sjtu.edu.cn
摘要:
克隆了百子莲(Agapanthus praecox)脱水素基因ApSK3上游2 195 bp的启动子序列,对百子莲不同组织和多种非生物胁迫与ABA处理的样品进行基因定量分析,构建多个ApSK3不同长度缺失的启动子片段与GUS基因融合的表达载体并转化拟南芥,以揭示ApSK3基因对不同逆境与激素信号的应答模式及顺式作用元件的调控功能。结果表明:ApSK3启动子序列包含多个与植物逆境、激素应答及生长发育相关的顺式作用元件,且ApSK3的表达具有组织特异性,果实中表达量最高,叶、根次之,花中最低;ApSK3对ABA信号与盐胁迫最敏感,其次为干旱、高渗和低温胁迫,对高温胁迫响应不明显。ApSK3-P::GUS 融合表达载体转化拟南芥,ApSK3启动子在拟南芥的整个生长发育过程中均具有较强的表达活性,幼苗根部的表达活性强于叶片,且随着果实的发育成熟启动子的活性明显增强。ApSK3不同长度缺失的启动子片段分析结果表明-2 175 ~-950 bp片段对启动子的活性起到重要的调控作用;多个顺式作用元件响应干旱胁迫;ApSK3-P通过两个ABRE元件共同响应ABA信号;-526 ~-533 bp的 ERE元件参与响应乙烯信号;-561 ~-567 bp的P-box元件响应了赤霉素信号,-2 175 ~-1 167 bp区域可以增强对GA的响应。研究结果证明ApSK3启动子可积极响应干旱、渗透、盐、低温、ABA、GA和乙烯信号;启动子上存在多个顺式作用元件响应干旱胁迫,ApSK3-P通过两个ABRE、ERE与P-box元件分别响应ABA、乙烯与赤霉素信号。
中图分类号:
杨天宸, 陈晓童, 吕可, 张荻. 百子莲脱水素基因ApSK3对逆境与激素信号的应答模式与调控机制[J]. 园艺学报, 2021, 48(8): 1565-1578.
YANG Tianchen, CHEN Xiaotong, LÜ Ke, ZHANG Di. Expression Pattern and Regulation Mechanism of ApSK3 Dehydrin (Agapanthus praecox)Response to Abiotic Stress and Hormone Signals[J]. Acta Horticulturae Sinica, 2021, 48(8): 1565-1578.
| 引物名称 | 序列(5′-3′) | 退火温度/℃ |
|---|---|---|
| Primer name | Primer sequence | Tm |
| Ap-Actin-S | CAGTGTCTGGATTGGAGG | 50.0 |
| Ap-Actin-A | TAGAAGCACTTCCTGTG | 50.0 |
| RT-ApSK3-S | AAGAGCCAAGAGGAGGTT | 55.0 |
| RT-ApSK3-A | CTTCTTCTCGCCGTCTTC | 55.0 |
| ApSK3-SP1 | TGTGGCCGGGGAGCTTCTGTTT | 61.4 |
| ApSK3-SP2 | ACGTCCTGTTCGGTTACCACGG | 61.4 |
| ApSK3-SP3 | CCACTTCCTCTTCGTCGCTCGA | 61.4 |
| ApSK3-SP2-1 | AGCTAGAGAAACGAATTATCACATCCCTC | 59.6 |
| ApSK3-SP2-2 | ACTTTCAAGCTCTCGACTCCGG | 59.5 |
| ApSK3-SP2-3 | CCCTCTCTCACTCACCTCCACA | 61.4 |
| Sp-2175-S | ATGACCATGATTACGCCAAGCTTGTGTTGCTATTTGTTAGAGAA | 56.0 |
| Sp-1167-S | ATGACCATGATTACGCCAAGCTTAAAGTAAAAAGAGCCAACACTTG | 55.0 |
| Sp-950-S | ATGACCATGATTACGCCAAGCTTCCACATCCGTCCATTAGTGC | 57.0 |
| Sp-646-S | ATGACCATGATTACGCCAAGCTTGGCAACATAATCATTAAGATACTGT | 57.0 |
| Sp-291-S | ATGACCATGATTACGCCAAGCTTGCTGATGGATAAGTAAAGAATAA | 55.0 |
| Sp-A | ACTGACCACCCGGGGATCCTTTTTTAATTAATTATAAACTTCAATG | 59.0 |
| pBI121-S | CGGCTCGTATGTTGTGTGGAATTG | 60.0 |
| pBI121-A | CGTTGGGGTTTCTACAGGACGTAA | 58.0 |
| Kana-S | TGGATTGCACGCAGGTTCTC | 58.0 |
| Kana-A | CTCGATGCGATGTTTCGCTT | 58.0 |
表1 引物序列
Table 1 Corresponding primer sequences
| 引物名称 | 序列(5′-3′) | 退火温度/℃ |
|---|---|---|
| Primer name | Primer sequence | Tm |
| Ap-Actin-S | CAGTGTCTGGATTGGAGG | 50.0 |
| Ap-Actin-A | TAGAAGCACTTCCTGTG | 50.0 |
| RT-ApSK3-S | AAGAGCCAAGAGGAGGTT | 55.0 |
| RT-ApSK3-A | CTTCTTCTCGCCGTCTTC | 55.0 |
| ApSK3-SP1 | TGTGGCCGGGGAGCTTCTGTTT | 61.4 |
| ApSK3-SP2 | ACGTCCTGTTCGGTTACCACGG | 61.4 |
| ApSK3-SP3 | CCACTTCCTCTTCGTCGCTCGA | 61.4 |
| ApSK3-SP2-1 | AGCTAGAGAAACGAATTATCACATCCCTC | 59.6 |
| ApSK3-SP2-2 | ACTTTCAAGCTCTCGACTCCGG | 59.5 |
| ApSK3-SP2-3 | CCCTCTCTCACTCACCTCCACA | 61.4 |
| Sp-2175-S | ATGACCATGATTACGCCAAGCTTGTGTTGCTATTTGTTAGAGAA | 56.0 |
| Sp-1167-S | ATGACCATGATTACGCCAAGCTTAAAGTAAAAAGAGCCAACACTTG | 55.0 |
| Sp-950-S | ATGACCATGATTACGCCAAGCTTCCACATCCGTCCATTAGTGC | 57.0 |
| Sp-646-S | ATGACCATGATTACGCCAAGCTTGGCAACATAATCATTAAGATACTGT | 57.0 |
| Sp-291-S | ATGACCATGATTACGCCAAGCTTGCTGATGGATAAGTAAAGAATAA | 55.0 |
| Sp-A | ACTGACCACCCGGGGATCCTTTTTTAATTAATTATAAACTTCAATG | 59.0 |
| pBI121-S | CGGCTCGTATGTTGTGTGGAATTG | 60.0 |
| pBI121-A | CGTTGGGGTTTCTACAGGACGTAA | 58.0 |
| Kana-S | TGGATTGCACGCAGGTTCTC | 58.0 |
| Kana-A | CTCGATGCGATGTTTCGCTT | 58.0 |

图1 ApSK3启动子的序列及顺式作用元件分布 起始密码子“ATG”的“A”被指定为“+ 1”。红色标注的为逆境胁迫及激素相关的元件,水平箭头表示方向。序列上方的垂直箭头表示不同缺失片段的起始点;蓝色核序列代表用于扩增缺失片段的特殊引物(Sp-S)。
Fig. 1 DNA sequence analysis of ApSK3 promoter and the description of cis-elements The“A”of the translation initiation code“ATG”of ApSK3 is designated as“+ 1”. These important cis-elements related with stress and hormone are red markerd. The horizontal arrows show their directions. The vertical arrows above the sequence indicate the start point of different deletion fragments;the blue nucleotide sequences represent special primers for amplifying deletion fragments(Sp-S).
| 类别 | 名称 | 描述 | 核心序列 | 数量 | 位置/bp |
|---|---|---|---|---|---|
| Category | Name | Description | Core Sequence | Number | Position |
| 结构元件 Structure elements | TATA-Box | 转录起始-30核心启动子元件 Core promoter element around-30 of transcription start | TATATA (5) | 16 | 123 ~-128,-1314 ~-1319, -1704 ~-1709, -1928 ~-1933, -1973 ~-1978 |
| TATAAA (1) | -595 ~-600 | ||||
| TATA (7) | 36 ~-39,-192~-195,-478 ~-481,-507 ~-510,-1208 ~-1211,-1753 ~-1756, -1778 ~-1781 | ||||
| TTTATA (3) | -15 ~-20,-1385 ~ -1390,-1459 ~-1464 | ||||
| CAAT-Box | 启动子和增强子区域调控元件 Common cis-acting element in promoter and enhancer regions | CAAT (9) | 15 | -89 ~-92,-822 ~-825, -991 ~-994,-1002 ~ -1005,-1661 ~-1664, -1713 ~-1716,-1840 ~ -1843,-1965 ~-1968, -2010 ~-2013 | |
| CAAAT (6) | -340 ~-344,-492 ~ -496,-1359 ~-1363, -1367 ~-1370,-1558 ~ -1561,-2068 ~-2072 | ||||
| 胁迫响应元件Abiotic stress responsive elements | ARE | 厌氧顺式调控元件 cis-Acting regulatory element essential for the anaerobic induction | AACCA | 2 | -584 ~-588, -1534 ~-1538 |
| MBS | 干旱诱导MYB结合位点 MYB binding site involved in drought-inducibility | CAACTG CAACAG | 2 | -1512 ~-1517, -319 ~-324 | |
| circadian | 昼夜顺式调控元件 Circadian-control element | CAAAGTTATC | 1 | -1628 ~-1637 | |
| TC-rich repeats | 防卫和胁迫响应顺式调控元件 cis-Acting element involved in defense and stress responsiveness | GTTTTCTAA ATTCTCTAAC | 2 | -1127 ~-1135 -2159 ~-2168 | |
| 激素响应元件Phytohor- mone responsive element | ABRE | 脱落酸响应顺式调控元件 Abscisic acid responsive element | CACGTG | 2 | -977 ~-982, -881 ~-886 |
| CGTCA-motif | 茉莉酸甲酯响应顺式调控元件 MeJA-responsive element | CGTCA | 2 | -165 ~-169, -1370 ~-1374 | |
| ERE | 乙烯响应顺式调控元件 Ethylene-responsive element | ATTTCAAA | 1 | -526 ~-533 | |
| P-box | 赤霉素响应顺式调控元件 Gibberellin-responsive element | CCTTTTG | 1 | -561 ~-567 | |
| TGA-box | 生长素响应顺式调控元件 Auxin-responsive element | CGGTGCAGT | 1 | -170 ~-178 | |
| 生长发育调控元件 Development-related elements | Skn-1 motif | 胚乳表达调控顺式作用元件 cis-Acting element involved in endosperm expression | GTCAT | 5 | -164 ~-168,-1005 ~-1009,-1785 ~-1789, -1956 ~-1960, -2023 ~-2027 |
| GCN4 motif | 胚乳表达调控顺式作用元件 cis-Acting element involved in endosperm expression | CAAGCCA | 1 | -1650 ~-1656 |
表2 ApSK3启动子中的顺式作用元件预测结果及功能列表
Table 2 Identification of cis-acting elements and functions in the ApSK3 promoter
| 类别 | 名称 | 描述 | 核心序列 | 数量 | 位置/bp |
|---|---|---|---|---|---|
| Category | Name | Description | Core Sequence | Number | Position |
| 结构元件 Structure elements | TATA-Box | 转录起始-30核心启动子元件 Core promoter element around-30 of transcription start | TATATA (5) | 16 | 123 ~-128,-1314 ~-1319, -1704 ~-1709, -1928 ~-1933, -1973 ~-1978 |
| TATAAA (1) | -595 ~-600 | ||||
| TATA (7) | 36 ~-39,-192~-195,-478 ~-481,-507 ~-510,-1208 ~-1211,-1753 ~-1756, -1778 ~-1781 | ||||
| TTTATA (3) | -15 ~-20,-1385 ~ -1390,-1459 ~-1464 | ||||
| CAAT-Box | 启动子和增强子区域调控元件 Common cis-acting element in promoter and enhancer regions | CAAT (9) | 15 | -89 ~-92,-822 ~-825, -991 ~-994,-1002 ~ -1005,-1661 ~-1664, -1713 ~-1716,-1840 ~ -1843,-1965 ~-1968, -2010 ~-2013 | |
| CAAAT (6) | -340 ~-344,-492 ~ -496,-1359 ~-1363, -1367 ~-1370,-1558 ~ -1561,-2068 ~-2072 | ||||
| 胁迫响应元件Abiotic stress responsive elements | ARE | 厌氧顺式调控元件 cis-Acting regulatory element essential for the anaerobic induction | AACCA | 2 | -584 ~-588, -1534 ~-1538 |
| MBS | 干旱诱导MYB结合位点 MYB binding site involved in drought-inducibility | CAACTG CAACAG | 2 | -1512 ~-1517, -319 ~-324 | |
| circadian | 昼夜顺式调控元件 Circadian-control element | CAAAGTTATC | 1 | -1628 ~-1637 | |
| TC-rich repeats | 防卫和胁迫响应顺式调控元件 cis-Acting element involved in defense and stress responsiveness | GTTTTCTAA ATTCTCTAAC | 2 | -1127 ~-1135 -2159 ~-2168 | |
| 激素响应元件Phytohor- mone responsive element | ABRE | 脱落酸响应顺式调控元件 Abscisic acid responsive element | CACGTG | 2 | -977 ~-982, -881 ~-886 |
| CGTCA-motif | 茉莉酸甲酯响应顺式调控元件 MeJA-responsive element | CGTCA | 2 | -165 ~-169, -1370 ~-1374 | |
| ERE | 乙烯响应顺式调控元件 Ethylene-responsive element | ATTTCAAA | 1 | -526 ~-533 | |
| P-box | 赤霉素响应顺式调控元件 Gibberellin-responsive element | CCTTTTG | 1 | -561 ~-567 | |
| TGA-box | 生长素响应顺式调控元件 Auxin-responsive element | CGGTGCAGT | 1 | -170 ~-178 | |
| 生长发育调控元件 Development-related elements | Skn-1 motif | 胚乳表达调控顺式作用元件 cis-Acting element involved in endosperm expression | GTCAT | 5 | -164 ~-168,-1005 ~-1009,-1785 ~-1789, -1956 ~-1960, -2023 ~-2027 |
| GCN4 motif | 胚乳表达调控顺式作用元件 cis-Acting element involved in endosperm expression | CAAGCCA | 1 | -1650 ~-1656 |

图2 百子莲不同组织中脱水素基因ApSK3的表达量 虚线表示Ap-actin(内参)表达水平。
Fig. 2 Expression level of ApSK3 in different tissues of Agapanthus praecox The dotted line indicated the expression level of Ap-actin(internal reference gene).
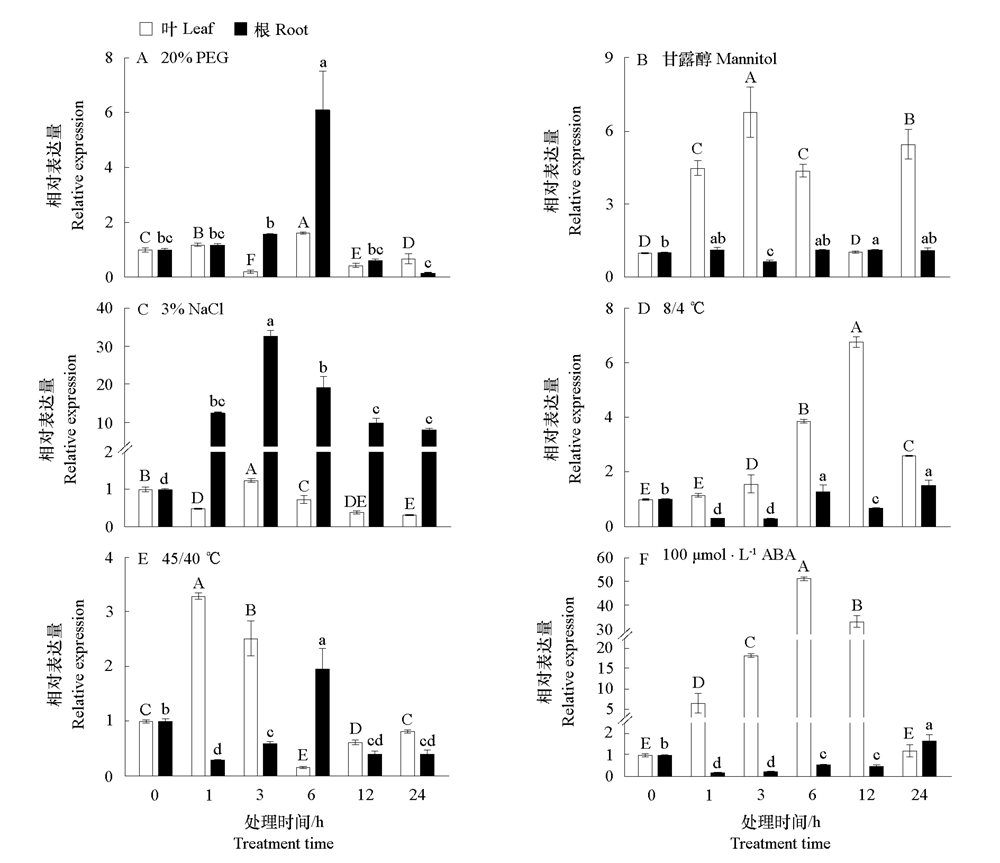
图4 百子莲在不同胁迫及ABA处理条件下根和叶片中ApSK3的表达分析 Ap-actin为内参基因。大写和小写字母分别表示在相同处理下,根和叶中不同时刻基因表达量间的差异显著性(P < 0.05)。
Fig. 4 Quantitative real-time PCR analysis of ApSK3 gene transcripts in Agapanthus praecox roots and leaves in response to various abiotic stresses and ABA treatment Ap-actin was used as internal reference gene. Values with different uppercase and lowercase letters are significantly different among samples in the roots and leaves,respectively.(P < 0.05).
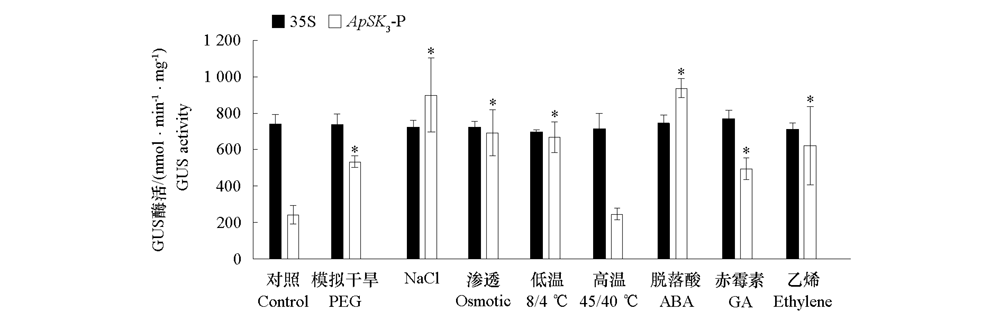
图5 不同胁迫、ABA、GA和乙烯处理后ApSK3-P转基因及空载拟南芥(35S启动子)的GUS活性分析 * 表示不同植物生长调节剂、胁迫处理组与对照间的差异显著(P < 0.05,α = 0.05)
Fig. 5 GUS activity analysis of transgenic Arabidopsis seedlings containing ApSK3-P or 35S promoter under abiotic,ABA,GA and Ethylene treatments Asterisks(*)indicate significant differences between ApSK3-P under different treatments and control.(P < 0.05,α = 0.05). Ap-actin was used as internal reference gene. Values with different uppercase and lowercase letters are significantly different among samples in the roots and leaves,respectively.
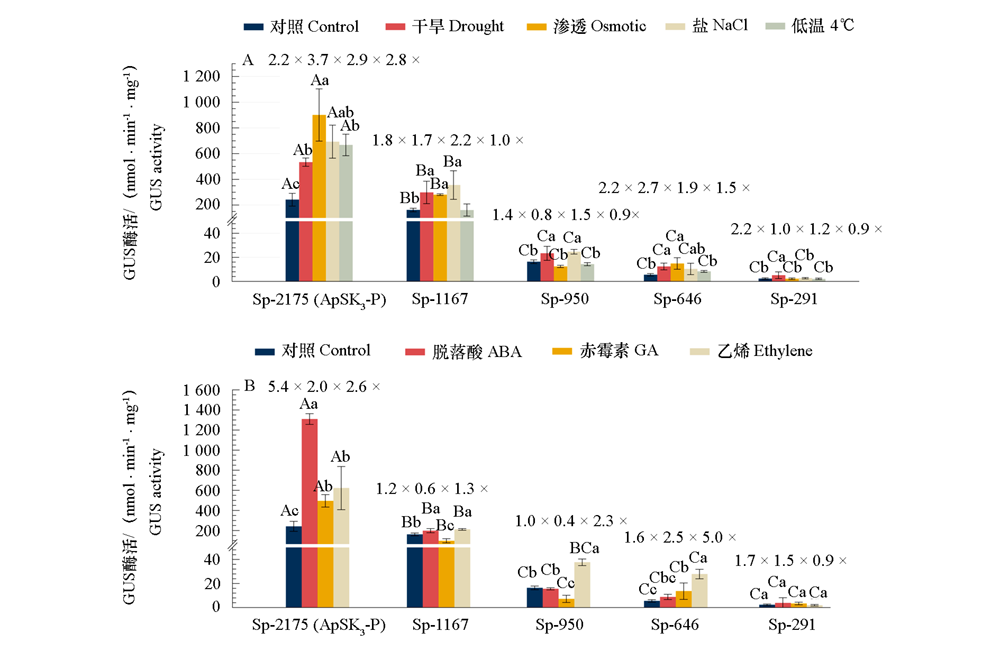
图7 转ApSK3启动子不同5'缺失片段的拟南芥在非生物胁迫(A)及外源ABA、GA、乙烯(B)处理下的GUS活性 不同大写字母代表不同启动子缺失片段在相同处理下的差异显著(P < 0.05);不同小写字母代表相同启动子缺失片段在不同处理下的差异显著(P < 0.05);柱上数字代表相同启动子缺失片段在不同处理下与对照的比值。
Fig. 7 GUS activity of transgenic Arabidopsis seedlings containing ApSK3 promoter and its 5' deletion constructs under abiotic(A)and ABA,GA,Ethylene(B)treatments Capital letters represent the significance of difference between the differernt ApSK3 promoters in the same treated condition;lower-case letters represent the significance among the same ApSK3 promoter under different treatments(P < 0.05,least significant difference test);numbers represent the fold of the same ApSK3 promoter under different treatments/Control.
| [1] |
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. 2003. Arabidopsis AtMYC2(bHLH)and AtMYB2(MYB)function as transcriptional activators in abscisic acid signaling. The Plant Cell, 15 (1):63-78.
doi: 10.1105/tpc.006130 URL |
| [2] |
Abedini R, GhaneGolmohammadi F, PishkamRad R, Pourabed E, Jafarnezhad A, Shobbar Z S, Shahbazi M. 2017. Plant dehydrins:shedding light on structure and expression patterns of dehydrin gene family in barley. Journal of Plant Research, 130 (4):747-763.
doi: 10.1007/s10265-017-0941-5 pmid: 28389925 |
| [3] | Allagulova C R, Gimalov F R, Shakirova F M, Vakhitov V A. 2003. The plant dehydrins:structure and putative functions. Biochemistry(Moscow), 68 (9):945-51. |
| [4] |
Bradford M M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72 (s 1-2):248-254.
doi: 10.1016/0003-2697(76)90527-3 URL |
| [5] |
Chen R G, Jing H, Guo W L, Wang S B, Ma F, Pan B G, Gong Z H. 2015. Silencing of dehydrin CaDHN1 diminishes tolerance to multiple abiotic stresses in Capsicum annuum L. Plant Cell Reports, 34 (12):2189-2200.
doi: 10.1007/s00299-015-1862-1 URL |
| [6] | Guo X Y, Liu D F, Chong K. 2018. Cold signaling in plants:insights into mechanisms and regulation. Journal of Integrative Plant Biology, 60 (9):7-18. |
| [7] |
Hernandez-Garcia C M, Finer J J. 2014. Identification and validation of promoters and cis-acting regulatory elements. Plant Science, 217-218 (1):109-119.
doi: 10.1016/j.plantsci.2013.12.007 URL |
| [8] |
Hobo T, Asada M, Kowyama Y, Hattori T. 1999. ACGT‐containing abscisic acid response element(ABRE)and coupling element 3(CE3)are functionally equivalent. The Plant Journal, 19 (6):679-689.
doi: 10.1046/j.1365-313x.1999.00565.x URL |
| [9] |
Hundertmark M, Hincha D K. 2008. LEA(Late Embryogenesis Abundant)proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics, 9 (1):118.
doi: 10.1186/1471-2164-9-118 URL |
| [10] |
Hwang S H, Lee I A, Yie S W, Hwang D J. 2008. Identification of an OsPR10a promoter region responsive to salicylic acid. Planta, 227 (5):1141-1150.
doi: 10.1007/s00425-007-0687-8 URL |
| [11] |
Jia F J, Qi S D, Li H, Liu P, Li P C, Wu C G, Zheng C C, Huang J G. 2014. Overexpression of Late Embryogenesis Abundant 14enhances Arabidopsis salt stress tolerance. Biochemical and Biophysical Research Communications, 454 (4):505-511.
doi: 10.1016/j.bbrc.2014.10.136 URL |
| [12] |
Kidokoro S, Watanabe K, Ohori T, Moriwaki T, Maruyama K, Mizoi J, Htwe N, Fujita Y, Sekita S, Shinozaki K, Yamaguchi-Shinozaki K. 2015. Soybean DREB1/CBF‐type transcription factors function in heat and drought as well as cold stress‐responsive gene expression. The Plant Journal, 81 (3):505-518.
doi: 10.1111/tpj.12746 pmid: 25495120 |
| [13] |
Kruger C, Berkowitz O, Stephan U W, Hell R. 2002. A metal-binding member of the late embryogenesis abundant protein family transports iron in the phloem of Ricinus communis L. Journal of Biological Chemistry, 277 (28):25062-25069.
doi: 10.1074/jbc.M201896200 URL |
| [14] |
Liang D, Xia H, Wu S, Ma F W. 2012. Genome-wide identification and expression profiling of dehydrin gene family in Malus domestica. Molecular Biology Reports, 39 (12):10759-10768.
doi: 10.1007/s11033-012-1968-2 URL pmid: 23053973 |
| [15] |
Liu H, Yu C Y, Li H X, Ouyang B, Wang T T, Zhang J H, Wang X, Ye Z B. 2015. Overexpression of ShDHN,a dehydrin gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses in tomato. Plant Science, 231:198-211.
doi: 10.1016/j.plantsci.2014.12.006 URL |
| [16] | Liu Yang. 2014. Isolation and functional analysis of LEA protein genes, ZmDHN13,ZmLEA3 and ZmLEA5C in Zea mays[Ph. D. Dissertation]. Tai’an:Shandong Agricultural University. (in Chinese) |
| 刘洋. 2014. 玉米LEA蛋白基因ZmDHN13、ZmLEA3和ZmLEA5C的分离与功能分析[博士论文]. 泰安: 山东农业大学. | |
| [17] |
Lü A M, Fan N N, Xie J P, Yuan S L, An Y, Zhou P. 2017. Expression of CdDHN4,a novel YSK2-type dehydrin gene from bermudagrass,responses to drought stress through the ABA-dependent signal pathway. Frontiers in Plant Science, 8:748.
doi: 10.3389/fpls.2017.00748 URL |
| [18] | Ma Jie, Liu Cui-fang, Li Ling-zhi, Xiang Jian-hua, Chen Xin-bo. 2012. Progress in research of dehydrin response to abiotic stress. Journal of Biology, 29 (1):71-74. (in Chinese) |
| 马杰, 刘翠芳, 李灵之, 向建华, 陈信波. 2012. 非生物胁迫下植物脱水素的研究进展. 生物学杂志, 29 (1):71-74. | |
| [19] |
Nakashima K, Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K. 1997. A nuclear gene,erd1,encoding a chloroplast-targeted Clp protease regulatory subunit homolog is not only induced by water stress but also developmentally up-regulated during senescence in Arabidopsis thaliana. The Plant Journal, 12 (4):851-861.
doi: 10.1046/j.1365-313X.1997.12040851.x URL |
| [20] |
Pellegrineschi A, Reynolds M, Pacheco M, Brito R M, Almeraya R, Yamaguchi-Shinozaki K, Hoisington D. 2004. Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome, 47 (3):493-500.
pmid: 15190366 |
| [21] | Qian Gang. 2007. Genotypic variability in sequences and expression of LEA2/LEA3 genes in Tibetan Hulless Barley,Hordeum vulgare ssp. vulgare,associated with resistance to water deficit[Ph. D. Dissertation]. Chengdu:Chengdu Institute of Biology,the Chinese Academy of Sciences. (in Chinese) |
| 钱刚. 2007. 不同抗旱性青稞LEA2/LEA3蛋白基因的克隆与表达[博士论文]. 成都: 中国科学院研究生院. | |
| [22] |
Robertson M, Cuming A C, Chandler P M. 1995. Sequence analysis and hormonal regulation of a dehydrin promoter from barley, Hordeum vulgare. Physiologia Plantarum, 94 (3):470-478.
doi: 10.1111/ppl.1995.94.issue-3 URL |
| [23] | Rorat T. 2007. Plant dehydrins——Tissue location,structure and function. Cellular & Molecular Biology Letters, 11 (4):536-556. |
| [24] |
Rorat T, Grygorowicz W J, Irzykowski W, Rey P. 2004. Expression of KS-type dehydrins is primarily regulated by factors related to organ type and leaf developmental stage during vegetative growth. Planta, 218 (5):878-885.
URL pmid: 14685858 |
| [25] |
Saibi W, Feki K, Ben Mahmoud R, Brini F. 2015. Durum wheat dehydrin(DHN-5)confers salinity tolerance to transgenic Arabidopsis plants through the regulation of proline metabolism and ROS scavenging system. Planta, 242 (5):1187-1194.
doi: 10.1007/s00425-015-2351-z URL |
| [26] | Swarup R, Parry G, Graham N, Allen T, Bennett M. 2002. Auxin cross-talk:integration of signalling pathways to control plant development. Plant Molecular Biology, 49 (3-4):411-426. |
| [27] | Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. 2000. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proceedings of the National Academy of Sciences of the United States of America, 97 (21):11632-11637. |
| [28] |
Vasil V, Marcotte W R,Jr, Rosenkrans L, Cocciolone S M, Vasil I K, Quatrano R S, McCarty D R. 1995. Overlap of Viviparous1( VP1)and abscisic acid response elements in the Em promoter:G-box elements are sufficient but not necessary for VP1 transactivation. Plant Cell, 7:1511-1518.
pmid: 8589631 |
| [29] |
Wang C H, Gao G, Cao S X, Xie Q J, Qi H Y. 2019. Isolation and functional validation of the CmLOX08 promoter associated with signalling molecule and abiotic stress responses in oriental melon,Cucumis melo var. makuwa Makino. BMC Plant Biology, 19 (1):75.
doi: 10.1186/s12870-019-1678-1 URL |
| [30] |
Wang H T, Zhang Y M, Xiao N, Zhang G, Wang F, Chen X Y, Fang R X. 2020a. Rice GERMIN-LIKE PROTEIN 2-1 functions in seed dormancy under the control of abscisic acid and gibberellic acid signaling pathways. Plant Physiology, 183 (3):1157-1170.
doi: 10.1104/pp.20.00253 URL |
| [31] | Wang Y D, Chen G J, Lei J J, Cao B H, Chen C M. 2020b. Identification and characterization of a LEA-like Gene,CaMF5,specifically expressed in the anthers of male-fertile Capsicum annuum. Horticultural Plant Journal, 26 (1):39-48. |
| [32] | Wu Qiong. 2019. Study of ABA-ethylene interaction on the regulation of cherry tomato fruit ripening[Ph. D. Dissertation]. Hangzhou:Zhejiang University. (in Chinese) |
| 吴琼. 2019. ABA-乙烯互作调控樱桃番茄果实成熟的效应与机理研究[博士论文]. 杭州:浙江大学. | |
| [33] |
Yamaguchi-Shinozaki K, Shinozaki K. 1994. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought,low-temperature,or high-salt stress. Plant Cell, 6 (2):251-264.
pmid: 8148648 |
| [34] |
Yamaguchi-Shinozaki K, Shinozaki K. 2005. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends in Plant Science, 10 (2):88-94.
pmid: 15708346 |
| [35] | Yang W B, Zhang L S, Lv H, Li H, Zhang Y N, Xu Y, Yu J N. 2015. The K-segments of wheat dehydrin WZY 2 are essential for its protective functions under temperature stress. Frontiers Plant Science, 6:406. |
| [36] |
Yang Z, Sheng J Y, Lv K, Ren L, Zhang D. 2019. Y2SK2 and SK3 type dehydrins from Agapanthus praecox can improve plant stress tolerance and act as multifunctional protectants. Plant Science, 284:143-160.
doi: 10.1016/j.plantsci.2019.03.012 URL |
| [37] |
Zhang H F, Liu S Y, Ma J H, Wang X K, Haq S U, Meng Y C, Zhang Y M, Chen R G. 2020. CaDHN4,a salt and cold stress-responsive dehydrin gene from pepper decreases abscisic acid sensitivity in Arabidopsis. International Journal of Molecular Sciences, 21 (1):26.
doi: 10.3390/ijms21010026 URL |
| [38] |
Zhao Y, Wang Y, Liu Q, Zhai Y, Zhao Y, Zhang M J. 2017. Cloning of a new LEA 1 gene promoter from soybean and functional analysis in transgenic tobacco. Plant Cell Tissue and Organ Culture, 130 (4-5):1-13.
doi: 10.1007/s11240-017-1199-2 URL |
| [39] |
Zhu K J, Wu Q J, Huang Y, Ye J L, Xu Q, Deng X X. 2020. Genome-wide characterization of cis-acting elements in the promoters of key carotenoid pathway genes from the main species of genus Citrus. Horticultural Plant Journal, 6 (6):385-395.
doi: 10.1016/j.hpj.2020.10.003 URL |
| [40] |
Zhu W N, Zhang D P, Lu X X, Zhang L S, Yu Z Y, Lv H, Zhang H M. 2014. Characterisation of an SKn-type dehydrin promoter from wheat and its responsiveness to various abiotic and biotic stresses. Plant Molecular Biology Reporter, 32 (3):664-678.
doi: 10.1007/s11105-013-0681-1 URL |
| [1] | 于婷婷, 李 欢, 宁源生, 宋建飞, 彭璐琳, 贾竣淇, 张玮玮, 杨洪强. 苹果GRAS全基因组鉴定及其对生长素的响应分析[J]. 园艺学报, 2023, 50(2): 397-409. |
| [2] | 袁馨, 徐云鹤, 张雨培, 单楠, 陈楚英, 万春鹏, 开文斌, 翟夏琬, 陈金印, 甘增宇. 猕猴桃后熟过程中ABA响应结合因子AcAREB1调控AcGH3.1的表达[J]. 园艺学报, 2023, 50(1): 53-64. |
| [3] | 徐小萍, 曹清影, 蔡柔荻, 官庆栩, 张梓浩, 陈裕坤, 徐涵, 林玉玲, 赖钟雄. 龙眼miR408与DlLAC12克隆及其在球形胚发生和非生物胁迫下的表达分析[J]. 园艺学报, 2022, 49(9): 1866-1882. |
| [4] | 贾鑫, 曾臻, 陈月, 冯慧, 吕英民, 赵世伟. 月季‘月月粉’RcDREB2A的克隆与表达分析[J]. 园艺学报, 2022, 49(9): 1945-1956. |
| [5] | 马维峰, 李艳梅, 马宗桓, 陈佰鸿, 毛娟. 苹果POD家族基因的鉴定与MdPOD15的功能分析[J]. 园艺学报, 2022, 49(6): 1181-1199. |
| [6] | 王丹, 王谧, 刘军, 周晓慧, 刘松瑜, 杨艳, 庄勇. 茄子U6启动子克隆及CRISPR/Cas9介导的基因编辑体系建立[J]. 园艺学报, 2022, 49(4): 791-800. |
| [7] | 宋放, 李子璇, 王策, 王志静, 何利刚, 蒋迎春, 吴黎明, 白福玺. 柑橘菌根信号受体蛋白基因LYK2的克隆及功能分析[J]. 园艺学报, 2022, 49(2): 281-292. |
| [8] | 周至铭, 杨佳宝, 张程, 曾令露, 孟晚秋, 孙黎. 向日葵LACS家族鉴定及响应非生物胁迫表达分析[J]. 园艺学报, 2022, 49(2): 352-364. |
| [9] | 黄仁维, 任迎虹, 祁伟亮, 曾睿, 刘欣宇, 邓彬艳. 桑树MaERF105-Like的克隆及其在干旱胁迫下的表达分析[J]. 园艺学报, 2022, 49(11): 2439-2448. |
| [10] | 谢思艺, 周承哲, 朱晨, 詹冬梅, 陈兰, 吴祖春, 赖钟雄, 郭玉琼. 茶树CsTIFY家族全基因组鉴定及非生物胁迫和激素处理中主要基因表达分析[J]. 园艺学报, 2022, 49(1): 100-116. |
| [11] | 梁志乐, 汪宽鸿, 杨静, 祝彪, 朱祝军. 硫代葡萄糖苷在十字花科植物应对非生物胁迫中的作用[J]. 园艺学报, 2022, 49(1): 200-220. |
| [12] | 马俊杰, 宋丽娜, 李乐, 马晓春, 靳磊, 徐伟荣. 山葡萄VaCBL6参与非生物胁迫和ABA途径的响应[J]. 园艺学报, 2021, 48(6): 1079-1093. |
| [13] | 邓泽宜, 宋想, 洪艳, 戴思兰. 启动子在观赏植物基因工程中的应用综述[J]. 园艺学报, 2021, 48(6): 1250-1264. |
| [14] | 蔡柔荻, 厉雪, 陈燕, 徐小萍, 陈晓慧, 赖钟雄, 林玉玲. 龙眼DRB家族全基因组鉴定及其表达分析[J]. 园艺学报, 2021, 48(5): 921-933. |
| [15] | 岳玲琦, 邢巧娟, 张晓兰, 梁雪, 王乾, 齐红岩. 光敏色素互作因子在植物抵御逆境胁迫中的作用研究进展[J]. 园艺学报, 2021, 48(4): 632-646. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司