
Acta Horticulturae Sinica ›› 2025, Vol. 52 ›› Issue (12): 3363-3372.doi: 10.16420/j.issn.0513-353x.2024-1040
• New Technology and New Method • Previous Articles Next Articles
YANG Jiali1,2, ZENG Zhilin1,2, ZHENG Zhe1,2, RAO Yufei1,2, HE Yanhong1,2,**( ), NING Guogui1,2,**(
), NING Guogui1,2,**( )
)
Received:2025-02-20
Revised:2025-05-21
Online:2025-12-25
Published:2025-12-20
Contact:
HE Yanhong, NING Guogui
YANG Jiali, ZENG Zhilin, ZHENG Zhe, RAO Yufei, HE Yanhong, NING Guogui. Establishment of an Efficient Genetic Transformation System Mediated by Agrobacterium tumefaciens in Pinguicula cyclosecta[J]. Acta Horticulturae Sinica, 2025, 52(12): 3363-3372.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.ahs.ac.cn/EN/10.16420/j.issn.0513-353x.2024-1040
| 卡那霉素/ (mg · L-1) Kan | 存活率/% Survival rate | 再生率/% Regeneration rate | 生长状态 Growth state |
|---|---|---|---|
| 0 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 无愈伤组织,叶片能直接再生不定芽 No callus tissue,leaves can directly regenerate adventitious buds |
| 10 | 100.00 ± 0.00 a | 85.33 ± 2.19 b | 出现白色愈伤,部分再生不定芽 White callus tissue appears,with some regenerating adventitious buds |
| 20 | 90.00 ± 6.67 b | 55.98 ± 4.60 c | 出现白色愈伤,部分再生不定芽 White callus tissue appears,with some regenerating adventitious buds |
| 30 | 81.11 ± 6.94 c | 25.56 ± 1.93 d | 出现白色愈伤,不定芽再生受抑制 White callus tissue appears,and the regeneration of adventitious buds is inhibited |
| 40 | 76.67 ± 6.67 cd | 17.78 ± 5.09 e | 无愈伤组织,不定芽再生受抑制 No callus tissue,and the regeneration of adventitious buds is inhibited |
| 50 | 71.11 ± 1.92 d | 2.22 ± 1.92 f | 无愈伤组织,不定芽再生受抑制 No callus tissue,and the regeneration of adventitious buds is inhibited |
| 100 | 38.89 ± 5.09 e | 0 f | 无愈伤组织,不能再生不定芽 No callus tissue,unable to regenerate adventitious buds |
| 200 | 23.33 ± 3.34 f | 0 f | 无愈伤组织,不能再生不定芽 No callus tissue,unable to regenerate adventitious buds |
| 300 | 16.52 ± 3.55 f | 0 f | 无愈伤组织,不能再生不定芽 No callus tissue,unable to regenerate adventitious buds |
Table 1 Effect of different Kan concentrations on leaf regeneration of Pinguicula cyclosecta
| 卡那霉素/ (mg · L-1) Kan | 存活率/% Survival rate | 再生率/% Regeneration rate | 生长状态 Growth state |
|---|---|---|---|
| 0 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 无愈伤组织,叶片能直接再生不定芽 No callus tissue,leaves can directly regenerate adventitious buds |
| 10 | 100.00 ± 0.00 a | 85.33 ± 2.19 b | 出现白色愈伤,部分再生不定芽 White callus tissue appears,with some regenerating adventitious buds |
| 20 | 90.00 ± 6.67 b | 55.98 ± 4.60 c | 出现白色愈伤,部分再生不定芽 White callus tissue appears,with some regenerating adventitious buds |
| 30 | 81.11 ± 6.94 c | 25.56 ± 1.93 d | 出现白色愈伤,不定芽再生受抑制 White callus tissue appears,and the regeneration of adventitious buds is inhibited |
| 40 | 76.67 ± 6.67 cd | 17.78 ± 5.09 e | 无愈伤组织,不定芽再生受抑制 No callus tissue,and the regeneration of adventitious buds is inhibited |
| 50 | 71.11 ± 1.92 d | 2.22 ± 1.92 f | 无愈伤组织,不定芽再生受抑制 No callus tissue,and the regeneration of adventitious buds is inhibited |
| 100 | 38.89 ± 5.09 e | 0 f | 无愈伤组织,不能再生不定芽 No callus tissue,unable to regenerate adventitious buds |
| 200 | 23.33 ± 3.34 f | 0 f | 无愈伤组织,不能再生不定芽 No callus tissue,unable to regenerate adventitious buds |
| 300 | 16.52 ± 3.55 f | 0 f | 无愈伤组织,不能再生不定芽 No callus tissue,unable to regenerate adventitious buds |
| 头孢霉素/(mg · L-1) Cef | 再生率/% Regeneration rate | 生长状态 Growth state | 农杆菌生长情况 Growth of bacteria |
|---|---|---|---|
| 0 | 100.00 ± 0.00 a | 叶片再生正常,不定芽生长健壮 Normal leaf regeneration, and the adventitious buds grow vigorously | 生长良好 Grow well |
| 100 | 100.00 ± 0.00 a | 叶片再生正常,不定芽细弱 Normal leaf regeneration,and the adventitious buds are thin and weak | 生长良好 Grow well |
| 200 | 96.67 ± 3.34 ab | 叶片再生正常,不定芽细弱 Normal leaf regeneration,and the adventitious buds are thin and weak | 生长受到抑制 Completely inhibited |
| 300 | 93.44 ± 3.18 b | 叶片再生正常,不定芽细弱 Normal leaf regeneration,and the adventitious buds are thin and weak | 生长受到抑制 Completely inhibited |
| 400 | 92.22 ± 3.84 c | 叶片再生受到抑制 Leaf regeneration is inhibited | 生长受到抑制 Completely inhibited |
Table 2 Effect of different Cef concentration on leaf regeneration of Pinguicula cyclosecta
| 头孢霉素/(mg · L-1) Cef | 再生率/% Regeneration rate | 生长状态 Growth state | 农杆菌生长情况 Growth of bacteria |
|---|---|---|---|
| 0 | 100.00 ± 0.00 a | 叶片再生正常,不定芽生长健壮 Normal leaf regeneration, and the adventitious buds grow vigorously | 生长良好 Grow well |
| 100 | 100.00 ± 0.00 a | 叶片再生正常,不定芽细弱 Normal leaf regeneration,and the adventitious buds are thin and weak | 生长良好 Grow well |
| 200 | 96.67 ± 3.34 ab | 叶片再生正常,不定芽细弱 Normal leaf regeneration,and the adventitious buds are thin and weak | 生长受到抑制 Completely inhibited |
| 300 | 93.44 ± 3.18 b | 叶片再生正常,不定芽细弱 Normal leaf regeneration,and the adventitious buds are thin and weak | 生长受到抑制 Completely inhibited |
| 400 | 92.22 ± 3.84 c | 叶片再生受到抑制 Leaf regeneration is inhibited | 生长受到抑制 Completely inhibited |
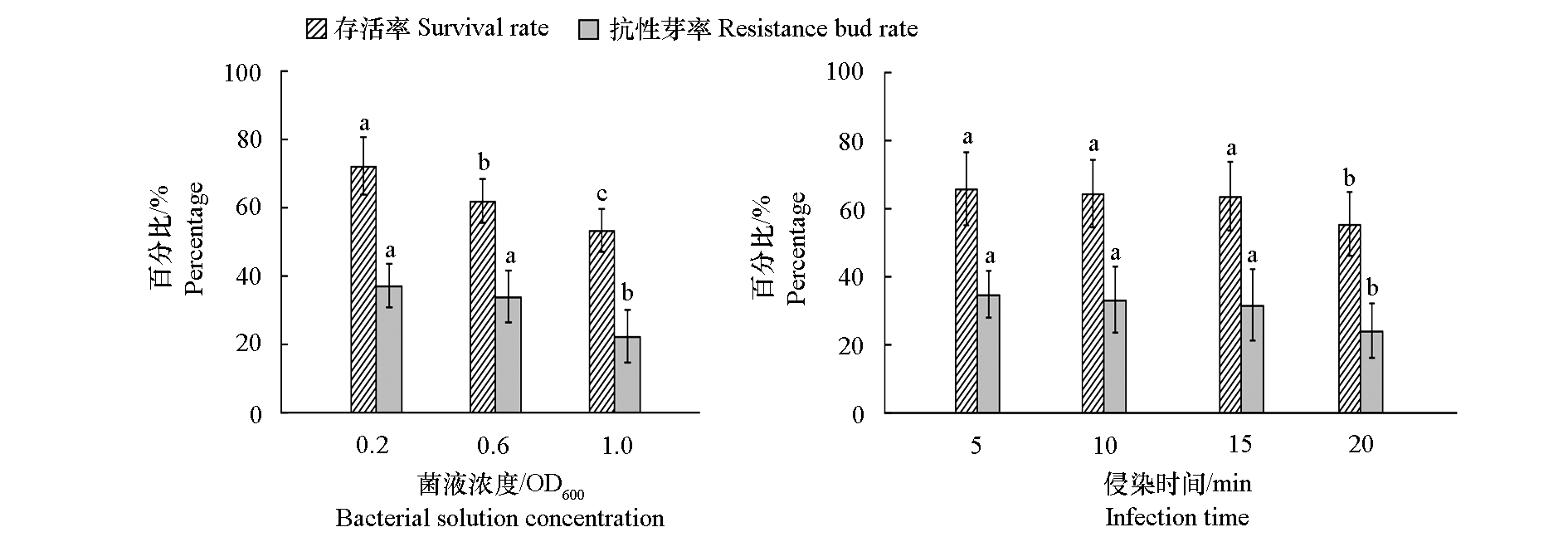
Fig. 1 Effect of bacterial solution concentration and infection time on the transformation of Pinguicula cyclosecta Different lowercase letters denote significant differences at 0.05 level. The same below
| 菌液浓度OD600 Bacterial solution concentration | 侵染时间/min Infection time | 存活率/% Survival rate | 抗性芽率/% Resistant bud rate | 阳性芽率/% Positive bud rate |
|---|---|---|---|---|
| 0.2 | 5 | 78.89 ± 5.09 a | 37.78 ± 6.94 abc | 0 b |
| 10 | 75.56 ± 9.62 ab | 41.11 ± 6.94 a | 0 b | |
| 15 | 72.22 ± 5.09 abc | 40.00 ± 5.77 ab | 7.78 ± 1.92 a | |
| 20 | 63.33 ± 6.67 cd | 31.11 ± 1.92 abcd | 1.11 ± 1.92 b | |
| 0.6 | 5 | 64.44 ± 1.92 bcd | 37.78 ± 8.39 abc | 0 b |
| 10 | 62.22 ± 1.92 cd | 35.56 ± 5.09 abc | 0 b | |
| 15 | 65.56 ± 8.39 bcd | 36.67 ± 8.8 2abc | 0 b | |
| 20 | 56.67 ± 8.82 de | 27.78 ± 6.94 bcd | 0 b | |
| 1.0 | 5 | 55.56 ± 3.85 de | 30.00 ± 3.33 abcd | 0 b |
| 10 | 56.67 ± 3.33 de | 25.56 ± 10.72 cde | 0 b | |
| 15 | 54.44 ± 8.39 de | 21.00 ± 5.09 de | 0 b | |
| 20 | 47.78 ± 6.94 e | 15.56 ± 1.92 e | 0 b |
Table 3 Effect of different infection conditions on transformation of Pinguicula cyclosecta
| 菌液浓度OD600 Bacterial solution concentration | 侵染时间/min Infection time | 存活率/% Survival rate | 抗性芽率/% Resistant bud rate | 阳性芽率/% Positive bud rate |
|---|---|---|---|---|
| 0.2 | 5 | 78.89 ± 5.09 a | 37.78 ± 6.94 abc | 0 b |
| 10 | 75.56 ± 9.62 ab | 41.11 ± 6.94 a | 0 b | |
| 15 | 72.22 ± 5.09 abc | 40.00 ± 5.77 ab | 7.78 ± 1.92 a | |
| 20 | 63.33 ± 6.67 cd | 31.11 ± 1.92 abcd | 1.11 ± 1.92 b | |
| 0.6 | 5 | 64.44 ± 1.92 bcd | 37.78 ± 8.39 abc | 0 b |
| 10 | 62.22 ± 1.92 cd | 35.56 ± 5.09 abc | 0 b | |
| 15 | 65.56 ± 8.39 bcd | 36.67 ± 8.8 2abc | 0 b | |
| 20 | 56.67 ± 8.82 de | 27.78 ± 6.94 bcd | 0 b | |
| 1.0 | 5 | 55.56 ± 3.85 de | 30.00 ± 3.33 abcd | 0 b |
| 10 | 56.67 ± 3.33 de | 25.56 ± 10.72 cde | 0 b | |
| 15 | 54.44 ± 8.39 de | 21.00 ± 5.09 de | 0 b | |
| 20 | 47.78 ± 6.94 e | 15.56 ± 1.92 e | 0 b |

Fig. 3 Fluorescence detection of seedlings(A)of Pinguicula cyclosecta,PCR detection of transgenic plants(B)and fluorescence detection of adults(C) WT:Wild type;T-GFP-1-T-GFP-7:Corresponding to the transgenic strains 1-7 in(B). M:Marker;0:Positive control;W:Negative control;1-16:Transgenic strains
| [1] |
|
|
安文杰, 张永侠, 原海燕. 2020. 红籽鸢尾瞬时表达体系的建立. 分子植物育种, 18(19):6359-6363.
|
|
| [2] |
|
| [3] |
|
|
陈莉, 马颖, 马锋旺, 梁东, 华智锐. 2007. 根癌农杆菌介导麝香百合遗传转化体系的建立. 西北植物学报,(7):1335-1340.
|
|
| [4] |
|
| [5] |
|
|
郭丽, 程征. 2019. 根癌农杆菌介导大岩桐遗传转化体系探究. 分子植物育种, 17:4638-4642.
|
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
|
孔维龙, 王翠, 但乃震, 李丹丹, 包满珠, 傅小鹏. 2018. 根癌农杆菌介导的香石竹遗传转化体系建立. 中国农业大学学报, 23(4):34-44.
|
|
| [10] |
|
|
刘晓东, 王婷婷, 刘群录, 陈嘉熙. 2014. 抗生素对非洲紫罗兰不定芽再生的影响. 北方园艺,(3):80-82.
|
|
| [11] |
|
| [12] |
|
| [13] |
|
|
宁国贵, 张润花. 2024. 食虫植物繁殖与栽培手册. 湖北: 湖北科学技术出版社:23-25.
|
|
| [14] |
|
| [15] |
|
| [16] |
|
|
任毅, 刘明时, 田联会, 田先华, 李智军. 2006. 太白山自然保护区生物多样性研究与管理. 北京: 中国林业出版社: 256.
|
|
| [17] |
|
|
苏秀娟, 王莉萍, 代培红, 陈全家, 曲延英. 2015. 英国薰衣草叶盘遗传转化体系的创建. 新疆农业科学, 52(3):517-522.
|
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
|
尹虹, 宋春丽, 马俊莲, 张子德, 唐霞. 2007. 根癌农杆菌介导的磨盘柿遗传转化体系的优化. 华北农学报, 22(2):56-59.
|
|
| [22] |
|
|
余晓敏, 王亚琴, 刘雨菡, 易庆平, 程文翰, 朱钰, 段枫, 张莉雪, 何燕红. 2023. 根癌农杆菌介导万寿菊遗传转化体系的建立. 植物学报, 58(5):760-769.
|
|
| [23] |
|
| [24] |
|
|
周蕴薇, 刘彧, 马欣, 高文杰, 何淼. 2016. 根癌农杆菌介导地被菊‘紫妍’遗传转化体系的建立. 西北农业学报, 25(12):1861-1869.
|
| [1] | DOU Xueting, ZHU Xi, ZHANG Ning, and SI Huaijun. Functional Analysis of StHY5 Associated with Low-Temperature Stress in Potato [J]. Acta Horticulturae Sinica, 2025, 52(7): 1745-1757. |
| [2] | WANG Wenlong, LIU Zhaokun, LU Wenjun, WANG Yaolong, LI Xiaofeng, ZHU Hongfang, LIU Tongkun, LI Ying, HOU Xilin, ZHANG Changwei. Enhancing Ascorbic Acid Content in Non-heading Chinese Cabbage Using Gene Editing Technology [J]. Acta Horticulturae Sinica, 2025, 52(5): 1317-1325. |
| [3] | WANG Zhongyi, LIU Yi, HU Bowen, ZHU Fan, LIU Feng, YANG Sha, XIONG Cheng, OU Lijun, DAI Xiongze, ZOU Xuexiao. Construction of a High-Efficiency Genetic Transformation System in Pepper Leveraging RUBY and CaREF1 [J]. Acta Horticulturae Sinica, 2025, 52(4): 1093-1104. |
| [4] | LI Min, LI Siyu, SHI Zihan, CHEN Shuang, XU Yan, LIU Guotian. Studies on the Efficiency of GRFs/GIFs for Genetic Transformation and Regeneration in Grapevine [J]. Acta Horticulturae Sinica, 2025, 52(1): 51-65. |
| [5] | YI Qian, ZHANG Manman, WANG Xiaoli, FENG Jipeng, ZHU Shiping, WANG Fusheng, ZHAO Xiaochun. CclSAUR49 Affects Growth and Limonoids Metabolism in Citrus [J]. Acta Horticulturae Sinica, 2024, 51(3): 479-494. |
| [6] | YANG Xue, HU Jinhong, LI Jingjing, ZHANG Yingcai, WANG Lingxia, LIANG Wenyu. Genetic Transformation of Lycium Barbarum TGA2 Gene in Arabidopsis Enhances Its Salt Tolerance [J]. Acta Horticulturae Sinica, 2024, 51(12): 2829-2842. |
| [7] | FENG Zhijuan, LIU Na, BU Yuanpeng, ZHANG Guwen, WANG Bin, GONG Yaming. Establishment of Agrobacterium rhizogenes-Mediated Genetic Transformation System in Vegetable Pea [J]. Acta Horticulturae Sinica, 2024, 51(10): 2439-2448. |
| [8] | LI Yamei, MA Fuli, ZHANG Shanqi, HUANG Jinqiu, CHEN Mengting, ZHOU Junyong, SUN Qibao, SUN Jun. Optimization of Jujube Callus Transformation System and Application of ZjBRC1 in Regulating ZjYUCCA Expression [J]. Acta Horticulturae Sinica, 2022, 49(4): 749-757. |
| [9] | SU Liyao, WANG Peiyu, JIANG Mengqi, HUANG Shuqi, XUE Xiaodong, LIU Mengyu, XIAO Xuechen, LAI Chunwang, ZHANG Zihao, CHEN Yukun, LAI Zhongxiong, LIN Yuling. The Activity Verification of pri-miR319a Encode Regulatory Peptide of Dimocarpus longan [J]. Acta Horticulturae Sinica, 2021, 48(5): 908-920. |
| [10] | LUO Hongyu, YANG Jiangwei, FENG Ya, ZHANG Huanhuan, LIU Shengyan, ZHANG Ning, SI Huaijun. The Effect of Stu-miR156 Silencing by STTM Technology on Potato Lateral Root Development [J]. Acta Horticulturae Sinica, 2021, 48(3): 531-538. |
| [11] | FU Yongyao1,Liu Jianling1,ZHU Yiyong1,XU Wenji1,Lei Meiyan2,and YANG Liping1,*. Construction of Transformation System and Integration of LrCCoAOMT Gene into Lilium lancifolium [J]. ACTA HORTICULTURAE SINICA, 2020, 47(7): 1345-1358. |
| [12] | CUI Huilin1,2,LI Zhiyuan2,FANG Zhiyuan2,YANG Limei2,ZHUANG Mu2,Lü Honghao2,LIU Yumei2,SONG Jianghua1,*,and ZHANG Yangyong2,*. Establishment and Application of YL-1 High-efficiency Genetic Transformation System in Cabbage(Brassica oleracea L. var. capitata) [J]. ACTA HORTICULTURAE SINICA, 2019, 46(2): 345-355. |
| [13] | LIU Hongjiu1,WEN Yanbin1,LIU Xiaoxue1,ZHAO Yongqiang1,HUANG Caiping2,and CHENG Zhihui1,*. A Review for Molecular Biology of Allium sativum [J]. ACTA HORTICULTURAE SINICA, 2018, 45(9): 1778-1790. |
| [14] | ZHANG Ruiteng1,2,Lü Jianchun1,ZHOU Mengdi1,LIU Jinglin3,LI Ren3,GUO Yangdong3,WANG Huaisong1,*,and FU Qiushi1,*. Expression and Function Analysis of CmGAS1 in Melon [J]. ACTA HORTICULTURAE SINICA, 2018, 45(10): 1929-1940. |
| [15] | DONG Jing,WANG Guixia,ZHONG Chuanfei,CHANG Linlin,SUN Jian,ZHANG Hongli,SUN Rui,SHI Kun,WEI Yongqing,and ZHANG Yuntao*. Studying Function of Alcohol Acyltransferase Gene FvAATW2 of Fragaria vesca by Over-expressing in Tobacco and Cultivated Strawberry [J]. ACTA HORTICULTURAE SINICA, 2018, 45(1): 41-50. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2012 Acta Horticulturae Sinica 京ICP备10030308号-2 国际联网备案号 11010802023439
Tel: 010-82109523 E-Mail: yuanyixuebao@126.com
Support by: Beijing Magtech Co.Ltd