
园艺学报 ›› 2021, Vol. 48 ›› Issue (4): 647-660.doi: 10.16420/j.issn.0513-353x.2020-0589
收稿日期:2021-01-26
出版日期:2021-04-25
发布日期:2021-04-29
通讯作者:
祝彪
E-mail:billzhu@zafu.edu.cn
基金资助:
WANG Kuanhong, ZHU Biao( ), ZHU Zhujun
), ZHU Zhujun
Received:2021-01-26
Online:2021-04-25
Published:2021-04-29
Contact:
ZHU Biao
E-mail:billzhu@zafu.edu.cn
摘要:
谷胱甘肽(GSH)是广泛存在于植物体内的一种重要的抗氧化物质,可以清除细胞代谢中产生的多余活性氧自由基,从而减少膜脂过氧化对细胞造成的伤害。谷胱甘肽二硫化物(GSSG)是GSH的氧化形式。近年来研究发现谷胱甘肽氧化还原对(GSH/GSSG)作为信号分子在植物应对逆境胁迫中起重要作用。本文综述了近年来GSH,尤其是 GSH/GSSG的作用,为后续通过调控GSH/GSSG调节植物抗性提供参考。
中图分类号:
汪宽鸿, 祝彪, 朱祝军. GSH/GSSG在植物应对非生物胁迫中的作用综述[J]. 园艺学报, 2021, 48(4): 647-660.
WANG Kuanhong, ZHU Biao, ZHU Zhujun. Review of the Role of GSH/GSSG in Plant Abiotic Stress Response[J]. Acta Horticulturae Sinica, 2021, 48(4): 647-660.
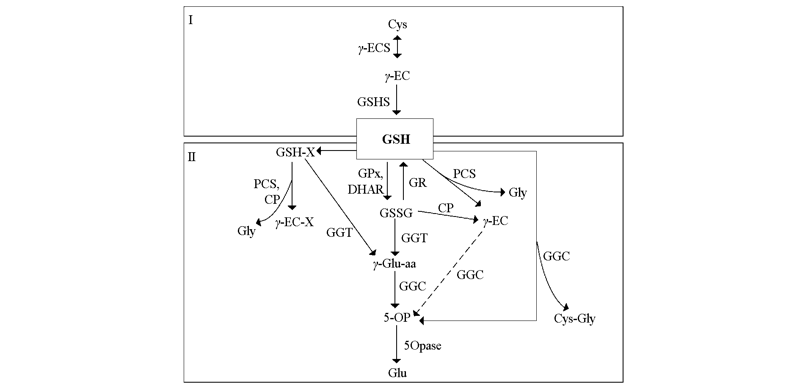
图1 植物中谷胱甘肽的代谢途径 I:合成途径;II:降解途径。参考Liu等(2015)和Hasanuzzaman等(2019)文献整合而成。
Fig. 1 Metabolic pathway of GSH in plants I:Biosynthesis pathway;II:Degradation pathway. Figure adapted and modified from Liu et al.2015 and Hasanuzzaman et al.2019.
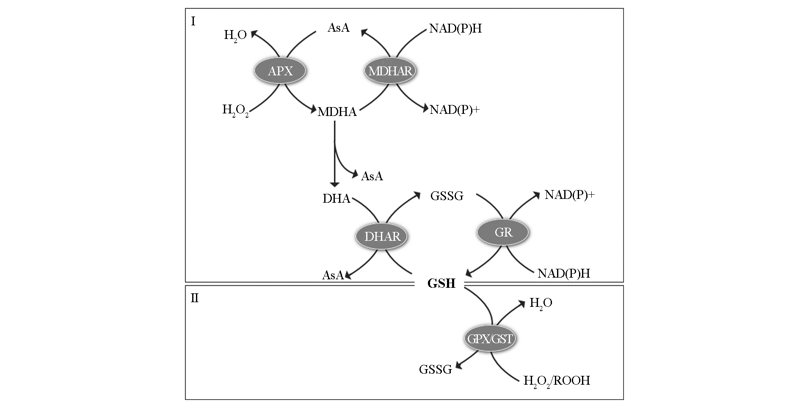
图2 谷胱甘肽氧化还原系统 I:抗坏血酸—谷胱甘肽循环;II:谷胱甘肽参与抗氧化酶系统。参考Hasanuzzaman等(2019)的文献整合而成。
Fig. 2 Overview of glutathione redox system I:Ascorbate-Glutathione cycle;II:Glutathione participants in anti-redox system. Figure adapted and modified from Hasanuzzaman et al.2019.
| 调控对象 Regulation object | 研究进展 Research progress | 植物种类 Species | 参考文献 Reference |
|---|---|---|---|
| SA | 调节SA介导的JA信号抑制 Regulate SA to depress JA signal | 拟南芥 Arabidopsis thaliana | Koornneef et al. |
| 通过抑制NPR1依赖的SA介导途径表达来减轻生物胁迫 Reduce biotic stress by inhibiting NPR1 dependent SA pathway expression | 烟草 Nicotiana tabacumL. | Ghanta et al. | |
| 诱导SA信号途径启动和SA积累及PR基因表达 Inducing SA signaling pathway initiation,SA accumulation and PR gene expression | 拟南芥 A.thaliana | Mhamdi et al. | |
| 在ICS1表达水平上调节SA积累,也独立于NPR1增加细胞内H2O2激活SA Regulate SA accumulation at ICS1 expression level,also increase intracellular H2O2 to activate SA in NPR1 independent pathway | 拟南芥 A.thaliana | Han et al. | |
| 通过SA和ET途径减弱AAL毒素 Attenuate AAL toxin through SA and ET pathways | 拟南芥 A.thaliana | Sultana et al. | |
| ET | 发现响应ET的GST基因簇 GST gene cluster responding to ET was found | 香石竹 Dianthus caryophyllus | Itzhaki et al. |
| 过表达GSH1,体细胞胚ACO转录增加 Overexpression of GSH1 increased ACO transcription in somatic embryos | 白云杉 Picea glauca | Stasolla et al. | |
| 外源施加GSSG,ET合成受阻 Exogenous GSSG inhibited ET synthesis | 白云杉 Picea glauca | Belmonte et al. | |
| 过量GSSG导致 SAM转录降低 Excessive GSSG decreased SAM transcription | 芸薹属 Brassica napus L. | Stasolla et al. | |
| 通过ACO和SAM的转录调控ET合成 Regulate ET synthesis through regulating transcription of ACO and SAM | 芸薹属 B.napusL. | Stasolla, | |
| 通过ACS和ACO的转录调控ET合成 Regulate ET synthesis through regulating transcription of ACS and ACO | 拟南芥 A.thaliana | Datta et al. | |
| 通过SA途径抑制ET合成,缓解AAL诱导的ROS应激 Inhibit ET synthesis through SA pathway and alleviate ROS stress induced by AAL | 拟南芥 A.thaliana | Sultana et al. | |
| JA | 在NPR1水平上调节SA和JA途径的拮抗作用 Regulate antagonism of SA and JA at NPR1 level | 拟南芥 A.thaliana | Spoel et al. |
| 参与MeJA信号传导 Participate in MeJA signal transduction | 拟南芥 A.thaliana | Akter et al. | |
| 独立于NPR1激活氧化应激触发的JA信号 Activate JA signal triggered by oxidative stress independently of NPR1 | 拟南芥 A.thaliana | Han et al. | |
| 增加JA应答基因的表达,增强对干旱和盐胁迫的耐受性 Increase expression of JA response gene,enhance tolerance to drought and salt stress | 拟南芥 A.thaliana | Cheng et al. | |
| GSH合成关键转录因子MYC2与在淹水胁迫中激活的JA信号相互作用 Key regulator of GSH biosynthesis MYC interact with JA signal in flooding stress | 拟南芥 A.thaliana | Yuan et al. | |
| ABA | GSH-ABA相互作用提高对非生物胁迫因子的耐受性 GSH-ABA interaction improves tolerance to abiotic stress factors | 拟南芥A.thaliana 小麦Triticum aestivumL. | Wei et al. |
| 外源GSH处理与ABA的积累有关 Exogenous GSH treatment is related to ABA accumulation | 拟南芥 A.thaliana | Chen et al. | |
| 激活ABA、生长素和JA的生物合成以及信号基因 Activate the biosynthesis of ABA,auxin and JA and signal response genes | 拟南芥 A.thaliana | Cheng et al. | |
| 影响ABA和MeJA含量,减少叶片气孔孔径 Affect the content of ABA and MeJA,and reduce the stomatal aperture | 拟南芥 A.thaliana | Akter et al. | |
| 与ABA、ET之间存在串扰 Crosstalk between ABA and ET | 拟南芥 A.thaliana | Kumar et al. | |
| NO | 与NO形成的GSNO连接ROS和活性氮信号通路 GSNO connects ROS and reactive nitrogen signaling pathway | 烟草 N. tabacum L. | Clark et al. |
| 镉胁迫激活GSNO信号,增强对镉胁迫的耐受性 Cadmium stress activated GSNO signal and enhanced tolerance to cadmium stress | 豌豆 Pisum sativumL. | Barroso et al. | |
| NO通过调节AsA-GSH循环来保护根系免受Al诱导的氧化胁迫 NO alleviates Al-induced oxidative damage through regulating the AsA-GSH cycle | 小麦 T. aestivumL. | Sun et al. | |
| 过量消耗GSH促进形成S-亚硝基硫醇,参与重金属解毒 Excessive consumption of GSH promotes the formation of S-nitrosothiol | 苎麻 Boehmeria nivea | Wang et al. | |
| 叶面喷施GSNO缓解水分亏缺对植物的氧化损伤 Foliar spraying of GSNO alleviates oxidative damage of plants under water deficit | 甘蔗 Saccharumspp. | Silveira et al. | |
| Ca2+ | 通过将Ca2+释放到胞浆传递信息参与应激诱导信号通路的早期部分Participate in early signal transduction events by stimulating calcium release into cytosol | 烟草 N.tabacum L. | Gomez et al. |
| 通过谷氧还原蛋白(Grx)影响Ca2+信号途径应对冷胁迫 Effect Ca2+ signaling pathway on cold stress through glutathione reducing protein | 水稻 Oryza sativaL. | Liu et al. |
表1 GSH对多种信号通路的调控
Table 1 Regulation of GSH on multiple signaling pathways
| 调控对象 Regulation object | 研究进展 Research progress | 植物种类 Species | 参考文献 Reference |
|---|---|---|---|
| SA | 调节SA介导的JA信号抑制 Regulate SA to depress JA signal | 拟南芥 Arabidopsis thaliana | Koornneef et al. |
| 通过抑制NPR1依赖的SA介导途径表达来减轻生物胁迫 Reduce biotic stress by inhibiting NPR1 dependent SA pathway expression | 烟草 Nicotiana tabacumL. | Ghanta et al. | |
| 诱导SA信号途径启动和SA积累及PR基因表达 Inducing SA signaling pathway initiation,SA accumulation and PR gene expression | 拟南芥 A.thaliana | Mhamdi et al. | |
| 在ICS1表达水平上调节SA积累,也独立于NPR1增加细胞内H2O2激活SA Regulate SA accumulation at ICS1 expression level,also increase intracellular H2O2 to activate SA in NPR1 independent pathway | 拟南芥 A.thaliana | Han et al. | |
| 通过SA和ET途径减弱AAL毒素 Attenuate AAL toxin through SA and ET pathways | 拟南芥 A.thaliana | Sultana et al. | |
| ET | 发现响应ET的GST基因簇 GST gene cluster responding to ET was found | 香石竹 Dianthus caryophyllus | Itzhaki et al. |
| 过表达GSH1,体细胞胚ACO转录增加 Overexpression of GSH1 increased ACO transcription in somatic embryos | 白云杉 Picea glauca | Stasolla et al. | |
| 外源施加GSSG,ET合成受阻 Exogenous GSSG inhibited ET synthesis | 白云杉 Picea glauca | Belmonte et al. | |
| 过量GSSG导致 SAM转录降低 Excessive GSSG decreased SAM transcription | 芸薹属 Brassica napus L. | Stasolla et al. | |
| 通过ACO和SAM的转录调控ET合成 Regulate ET synthesis through regulating transcription of ACO and SAM | 芸薹属 B.napusL. | Stasolla, | |
| 通过ACS和ACO的转录调控ET合成 Regulate ET synthesis through regulating transcription of ACS and ACO | 拟南芥 A.thaliana | Datta et al. | |
| 通过SA途径抑制ET合成,缓解AAL诱导的ROS应激 Inhibit ET synthesis through SA pathway and alleviate ROS stress induced by AAL | 拟南芥 A.thaliana | Sultana et al. | |
| JA | 在NPR1水平上调节SA和JA途径的拮抗作用 Regulate antagonism of SA and JA at NPR1 level | 拟南芥 A.thaliana | Spoel et al. |
| 参与MeJA信号传导 Participate in MeJA signal transduction | 拟南芥 A.thaliana | Akter et al. | |
| 独立于NPR1激活氧化应激触发的JA信号 Activate JA signal triggered by oxidative stress independently of NPR1 | 拟南芥 A.thaliana | Han et al. | |
| 增加JA应答基因的表达,增强对干旱和盐胁迫的耐受性 Increase expression of JA response gene,enhance tolerance to drought and salt stress | 拟南芥 A.thaliana | Cheng et al. | |
| GSH合成关键转录因子MYC2与在淹水胁迫中激活的JA信号相互作用 Key regulator of GSH biosynthesis MYC interact with JA signal in flooding stress | 拟南芥 A.thaliana | Yuan et al. | |
| ABA | GSH-ABA相互作用提高对非生物胁迫因子的耐受性 GSH-ABA interaction improves tolerance to abiotic stress factors | 拟南芥A.thaliana 小麦Triticum aestivumL. | Wei et al. |
| 外源GSH处理与ABA的积累有关 Exogenous GSH treatment is related to ABA accumulation | 拟南芥 A.thaliana | Chen et al. | |
| 激活ABA、生长素和JA的生物合成以及信号基因 Activate the biosynthesis of ABA,auxin and JA and signal response genes | 拟南芥 A.thaliana | Cheng et al. | |
| 影响ABA和MeJA含量,减少叶片气孔孔径 Affect the content of ABA and MeJA,and reduce the stomatal aperture | 拟南芥 A.thaliana | Akter et al. | |
| 与ABA、ET之间存在串扰 Crosstalk between ABA and ET | 拟南芥 A.thaliana | Kumar et al. | |
| NO | 与NO形成的GSNO连接ROS和活性氮信号通路 GSNO connects ROS and reactive nitrogen signaling pathway | 烟草 N. tabacum L. | Clark et al. |
| 镉胁迫激活GSNO信号,增强对镉胁迫的耐受性 Cadmium stress activated GSNO signal and enhanced tolerance to cadmium stress | 豌豆 Pisum sativumL. | Barroso et al. | |
| NO通过调节AsA-GSH循环来保护根系免受Al诱导的氧化胁迫 NO alleviates Al-induced oxidative damage through regulating the AsA-GSH cycle | 小麦 T. aestivumL. | Sun et al. | |
| 过量消耗GSH促进形成S-亚硝基硫醇,参与重金属解毒 Excessive consumption of GSH promotes the formation of S-nitrosothiol | 苎麻 Boehmeria nivea | Wang et al. | |
| 叶面喷施GSNO缓解水分亏缺对植物的氧化损伤 Foliar spraying of GSNO alleviates oxidative damage of plants under water deficit | 甘蔗 Saccharumspp. | Silveira et al. | |
| Ca2+ | 通过将Ca2+释放到胞浆传递信息参与应激诱导信号通路的早期部分Participate in early signal transduction events by stimulating calcium release into cytosol | 烟草 N.tabacum L. | Gomez et al. |
| 通过谷氧还原蛋白(Grx)影响Ca2+信号途径应对冷胁迫 Effect Ca2+ signaling pathway on cold stress through glutathione reducing protein | 水稻 Oryza sativaL. | Liu et al. |
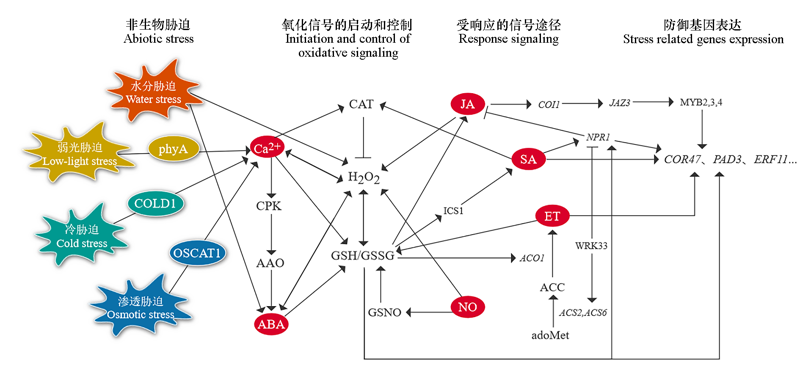
图3 GSH/GSSG参与非生物胁迫信号传导途径的一般模型 参考Zhu(2016)和Dutta(2018)的文献整合而成。
Fig. 3 A general model for the involvement of the GSH/GSSG in abiotic stress signal transduction pathway Figure adapted and modified from Zhu,2016 and Dutta,2018.
| [1] |
Akter N, Sobahan M, Uraji M, Ye W, Hossain M, Mori I, Nakamura Y, Murata Y. 2012. Effects of depletion of glutathione on abscisic acid- and methyl jasmonate-induced stomatal closure in Arabidopsis thaliana. Bioscience,Biotechnology,and Biochemistry, 76:2032-2037.
doi: 10.1271/bbb.120384 URL |
| [2] |
Akter N, Sobahan M A, Hossain M A, Uraji M, Nakamura Y, Mori I C, Murata Y. 2010. The involvement of intracellular glutathione in methyl jasmonate signaling in Arabidopsis guard cells. Bioscience,Biotechnology,and Biochemistry, 74:2504-2506.
doi: 10.1271/bbb.100513 URL |
| [3] | Bachhawat A K, Kaur A. 2017. Glutathione degradation. Antioxidants & Redox Signaling, 27:1200-1216. |
| [4] |
Barroso J B, Corpas F J, Carreras A, Rodríguez-Serrano M, Esteban F J, Fernández-Ocaña A, Chaki M, Romero-Puertas M C, Valderrama R, Sandalio L M, Del Río L A. 2006. Localization of S-nitrosoglutathione and expression of S-nitrosoglutathione reductase in pea plants under cadmium stress. Journal of Experimental Botany, 57:1785-1793.
doi: 10.1093/jxb/erj175 URL |
| [5] |
Begara-Morales J C, Chaki M, Valderrama R, Sánchez Calvo B, Mata Pérez C, Padilla M N, Corpas F J, Barroso J B. 2018. Nitric oxide buffering and conditional nitric oxide release in stress response. Journal of Experimental Botany, 69:3425-3438.
doi: 10.1093/jxb/ery072 pmid: 29506191 |
| [6] |
Bela K, Horváth E, Gallé Á, Szabados L, Tari I, Csiszár J. 2015. Plant glutathione peroxidases:emerging role of the antioxidant enzymes in plant development and stress responses. Journal of Plant Physiology, 176:192-201.
doi: 10.1016/j.jplph.2014.12.014 URL |
| [7] |
Belmonte M F, Donald G, Reid D M, Yeung E C, Stasolla C. 2005. Alterations of the glutathione redox state improve apical meristem structure and somatic embryo quality in white spruce (Picea glauca). Journal of Experimental Botany, 56:2355-2364.
doi: 10.1093/jxb/eri228 URL |
| [8] |
Blum R, Beck A, Korte A, Stengel A, Letzel T, Lendzian K, Grill E. 2007. Function of phytochelatin synthase in catabolism of glutathione-conjugates. The Plant Journal, 49:740-749.
doi: 10.1111/j.1365-313X.2006.02993.x URL |
| [9] | Bossio E, Díaz Paleo A, Del Vas M, Baroli I, Acevedo A, Ríos R D. 2013. Silencing of the glutathione biosynthetic pathway inhibits somatic embryogenesis in wheat. Plant Cell,Tissue and Organ Culture(PCTOC), 112:239-248. |
| [10] |
Chen J H, Jiang H W, Hsieh E J, Chen H Y, Chien C T, Hsieh H L, Lin T P. 2012. Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiology, 158:340-351.
doi: 10.1104/pp.111.181875 URL |
| [11] |
Cheng M C, Ko K, Chang W L, Kuo W C, Chen G H, Lin T P. 2015. Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. The Plant Journal, 83:926-939.
doi: 10.1111/tpj.2015.83.issue-5 URL |
| [12] |
Clark D, Durner J, Navarre R, Klessig D. 2001. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Molecular Plant-microbe Interactions, 13:1380-1384.
doi: 10.1094/MPMI.2000.13.12.1380 URL |
| [13] |
Datta R, Chattopadhyay S. 2015. Changes in the proteome of pad2-1,a glutathione depleted Arabidopsis mutant,during pseudomonas syringae infection. Journal of Proteomics, 126:82-93.
doi: 10.1016/j.jprot.2015.04.036 URL |
| [14] | Datta R, Kumar D, Sultana A, Hazra S, Bhattacharyya D, Chattopadhyay S. 2015. Glutathione regulates 1-aminocyclopropane-1-carboxylate synthase transcription via WRKY33 and 1-aminocyclopropane-1-carboxylate oxidase by modulating messenger RNA stability to induce ethylene synthesis during stress. Plant Physiology, 169:2963-2981. |
| [15] | Dutta R. 2018. Glutathione as a crucial modulator of phytohormone signalling during pathogen defence in plants. Proceedings of the Indian National Science Academy, 97:581-597. |
| [16] |
Foyer C H, Noctor G. 2011. Ascorbate and glutathione:the heart of the redox hub. Plant Physiology, 155:2-18.
doi: 10.1104/pp.110.167569 URL |
| [17] | Ghanta S, Bhattacharyya D, Chattopadhyay S. 2011. Glutathione signaling acts through NPR1-dependent SA-mediated pathway to mitigate biotic stress. Plant Signaling & Behavior, 6:607-609. |
| [18] |
Gill S S, Anjum N A, Hasanuzzaman M, Gill R, Trivedi D K, Ahmad I, Pereira E, Tuteja N. 2013. Glutathione and glutathione reductase:a boon in disguise for plant abiotic stress defense operations. Plant Physiology and Biochemistry, 70:204-212.
doi: 10.1016/j.plaphy.2013.05.032 URL |
| [19] |
Gomez L D, Noctor G, Knight M R, Foyer C H. 2004. Regulation of calcium signalling and gene expression by glutathione. Journal of Experimental Botany, 55:1851-1859.
doi: 10.1093/jxb/erh202 URL |
| [20] | Han Min, Cao Bi li, Liu Shu lin, Xu Kun 2019. Effects of root stock and scion interactions on ascorbate-glutathione cycle in tomato seedlings under low temperature stress. Acta Horticulturae Sinica, 46 (1):65-73. (in Chinese) |
| 韩敏, 曹逼力, 刘树森, 徐坤. 2019. 低温胁迫下番茄幼苗根穗互作对其抗坏血酸—谷胱甘肽循环的影响. 园艺学报, 46 (1):65-73. | |
| [21] |
Han Y, Chaouch S, Mhamdi A, Queval G, Zechmann B, Noctor G. 2012. Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid Redox Signal, 18:2106-2121.
doi: 10.1089/ars.2012.5052 URL |
| [22] | Han Y, Mhamdi A, Chaouch S, Noctor G. 2013. Regulation of basal and oxidative stress-triggered jasmonic acid-related gene expression by glutathione. Plant,Cell & Environment, 36:1135-1146. |
| [23] |
Hasanuzzaman M, Bhuyan M, Zulfiqar F, Raza A, Mohsin S M, Mahmud J A, Fujita M, Fotopoulos V. 2020. Reactive oxygen species and antioxidant defense in plants under abiotic stress:revisiting the crucial role of a universal defense regulator. Antioxidants, 9:681.
doi: 10.3390/antiox9080681 URL |
| [24] | Hasanuzzaman M, Bhuyan M H M B, Anee T I, Parvin K, Nahar K, Mahmud J A, Fujita M. 2019. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants(Basel), 8:384. |
| [25] |
Hasanuzzaman M, Nahar K, Anee T I, Fujita M. 2017. Glutathione in plants:biosynthesis and physiological role in environmental stress tolerance. Physiology and Molecular Biology of Plants, 23:249-268.
doi: 10.1007/s12298-017-0422-2 pmid: 28461715 |
| [26] |
He J, Li H, Ma C, Zhang Y, Polle A, Rennenberg H, Cheng X, Luo Z-B. 2015. Overexpression of bacterial γ-glutamylcysteine synthetase mediates changes in cadmium influx,allocation and detoxification in poplar. New Phytologist, 205:240-254.
doi: 10.1111/nph.2014.205.issue-1 URL |
| [27] |
Hsieh H L, Okamoto H. 2014. Molecular interaction of jasmonate and phytochrome A signalling. Journal of Experimental Botany, 65:2847-2857.
doi: 10.1093/jxb/eru230 URL |
| [28] |
Itzhaki H, Woodson W R. 1993. Characterization of an ethylene-responsive glutathione S-transferase gene cluster in carnation. Plant Molecular Biology, 22:43-58.
doi: 10.1007/BF00038994 URL |
| [29] | Jiang M, Zhang J. 2003. Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defence in leaves of maize seedlings. Plant Cell & Environment, 26:929-939. |
| [30] |
Kapoor D, Singh S, Kumar V, Romero R, Prasad R, Singh J. 2019. Antioxidant enzymes regulation in plants in reference to reactive oxygen species(ROS)and reactive nitrogen species(RNS). Plant Gene, 19:100182.
doi: 10.1016/j.plgene.2019.100182 URL |
| [31] |
Koornneef A, Leon-Reyes A, Ritsema T, Verhage A, Den Otter F C, van Loon L C, Pieterse C M J. 2008. Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiology, 147:1358-1368.
doi: 10.1104/pp.108.121392 URL |
| [32] |
Kranner I, Grill D. 1993. Content of low-molecular-weight thiols during the imbibition of pea seeds. Physiologia Plantarum, 88:557-562.
doi: 10.1111/ppl.1993.88.issue-4 URL |
| [33] |
Kudełko K, Gaj M D. 2019. Glutathione(GSH)induces embryogenic response in in vitro cultured explants of Arabidopsis thaliana via auxin-related mechanism. Plant Growth Regulation, 89:25-36.
doi: 10.1007/s10725-019-00514-1 URL |
| [34] |
Kumar D, Hazra S, Datta R, Chattopadhyay S. 2016. Transcriptome analysis of Arabidopsis mutants suggests a crosstalk between ABA,ethylene and GSH against combined cold and osmotic stress. Scientific Reports, 6:36867-36867.
doi: 10.1038/srep36867 URL |
| [35] |
Kumar S, Trivedi P K. 2018. Glutathione S-transferases:role in combating abiotic stresses including arsenic detoxification in plants. Frontiers in Plant Science, 9:751.
doi: 10.3389/fpls.2018.00751 URL |
| [36] |
Kwon E, Feechan A, Yun B W, Hwang B H, Pallas J A, Kang J G, Loake G J. 2012. AtGSNOR1 function is required for multiple developmental programs in Arabidopsis. Planta, 236:887-900.
doi: 10.1007/s00425-012-1697-8 URL |
| [37] |
Lai Z, Vinod K M, Zheng Z, Fan B, Chen Z. 2008. Roles of Arabidopsis WRKY3 and WRKY 4 transcription factors in plant responses to pathogens. BMC Plant Biology, 8:68.
doi: 10.1186/1471-2229-8-68 URL |
| [38] |
Laxa M, Liebthal M, Telman W, Chibani K, Dietz K J. 2019. The role of the plant antioxidant system in drought tolerance. Antioxidants, 8:94.
doi: 10.3390/antiox8040094 URL |
| [39] | Liu Pan, Geng Xingmin, Zhao Hui. 2020. Subcellular distribution and responses of antioxidant systems in leaves of three rhododendron cultivars under alkali stress. Acta Horticulturae Sinica, 47 (5):916-926. (in Chinese) |
| 刘攀, 耿兴敏, 赵晖. 2020. 碱胁迫下杜鹃花抗氧化体系的响应及亚细胞分布. 园艺学报, 47 (5):916-926. | |
| [40] |
Liu X, Zhang S, Whitworth R J, Stuart J J, Chen M-S. 2015. Unbalanced activation of glutathione metabolic pathways suggests potential involvement in plant defense against the gall midge Mayetiola destructor in wheat. Scientific Reports, 5:8092.
doi: 10.1038/srep08092 URL |
| [41] |
Liu W, Zheng C, Chen J, Qiu J, Huang Z, Wang Q, Ye Y. 2019. Cold acclimation improves photosynthesis by regulating the ascorbate-glutathione cycle in chloroplasts of Kandelia obovata. Journal of Forestry Research, 30:755-765.
doi: 10.1007/s11676-018-0791-6 URL |
| [42] |
Liu Y, Xu C, Zhu Y, Zhang L, Chen T, Zhou F, Chen H, Lin Y. 2018. The calcium-dependent kinase OsCPK24 functions in cold stress responses in rice. Journal of Integrative Plant Biology, 60:173-188.
doi: 10.1111/jipb.12614 URL |
| [43] | Locato V, Cimini S, De Gara L. 2017. Glutathione as a key player in plant abiotic stress responses and tolerance//Hossain M A,Mostofa M G,Diaz V P. Glutathione in plant growth,development,and stress tolerance. Cham:Springer International Publishing. |
| [44] |
Ma Y, Dai X, Xu Y, Luo W, Zheng X, Zeng D, Pan Y, Lin X, Liu H, Zhang D, Xiao J, Guo X, Xu S, Niu Y, Jin J, Zhang H, Xu X, Li L, Wang W, Qian Q, Ge S, Chong K. 2015. COLD 1 confers chilling tolerance in rice. Cell, 160:1209-1221.
doi: 10.1016/j.cell.2015.01.046 URL |
| [45] |
Marty L, Bausewein D, Müller C, Bangash S A K, Moseler A, Schwarzländer M, Müller-Schüssele S J, Zechmann B, Riondet C, Balk J, Wirtz M, Hell R, Reichheld J P, Meyer A J. 2019. Arabidopsis glutathione reductase 2 is indispensable in plastids,while mitochondrial glutathione is safeguarded by additional reduction and transport systems. New Phytologist, 224:1569-1584.
doi: 10.1111/nph.v224.4 URL |
| [46] |
Mhamdi A, Hager J, Chaouch S, Queval G, Han Y, Taconnat L, Saindrenan P, Gouia H, Issakidis-Bourguet E, Renou J P, Noctor G. 2010. Arabidopsis GLUTATHIONE REDUCTASE1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiology, 153:1144-1160.
doi: 10.1104/pp.110.153767 pmid: 20488891 |
| [47] |
Mou Z, Fan W, Dong X. 2003. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell, 113:935-944.
doi: 10.1016/S0092-8674(03)00429-X URL |
| [48] | Müller S. 2015. Role and regulation of glutathione metabolism in Plasmodium falciparum. Molecules(Basel,Switzerland), 20:10511-10534. |
| [49] |
Noctor G, Foyer C H. 1998. Ascorbate and glutathione:keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology, 49:249-279.
doi: 10.1146/annurev.arplant.49.1.249 URL |
| [50] | Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foryer C H. 2012. Glutathione in plants:an integrated overview. Plant Cell & Environment, 35:454-484. |
| [51] | Ntuli T M. 2014. The role-activity and/or processing-of (re) active oxygen species in desiccation sensitivity and/or tolerance,development,dormancy and/or germination in seeds. Journal of Horticulture, 1:1-10. |
| [52] |
Preuss M L, Cameron J C, Berg R H, Jez J M. 2014. Immunolocalization of glutathione biosynthesis enzymes in Arabidopsis thaliana. Plant Physiology and Biochemistry, 75:9-13.
doi: 10.1016/j.plaphy.2013.11.027 URL |
| [53] | Ranty B, Aldon D, Cotelle V, Galaud J P, Thuleau P, Mazars C. 2016. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Frontiers in Plant Science, 7:327. |
| [54] | Rao A S V C, Reddy A R. 2008. Glutathione reductase:a putative redox regulatory system in plant cells//Khan N A,Singh S,Umar S ed. Sulfur assimilation and abiotic stress in plants. Berlin:Heidelberg: Springer Berlin Heidelberg. |
| [55] | Schnaubelt, Daniel. 2013. The roles of glutathione in the control of plant growth,development and signalling in Arabidopsis thaliana[Ph. D. Dissertation]. Leeds:University of Leeds. |
| [56] |
Shan C, Zhou Y, Liu M. 2015. Nitric oxide participates in the regulation of the ascorbate-glutathione cycle by exogenous jasmonic acid in the leaves of wheat seedlings under drought stress. Protoplasma, 252:1397-1405.
doi: 10.1007/s00709-015-0756-y URL |
| [57] |
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K. 2002. Regulation and function of ascorbate peroxidase isoenzymes. Journal of Experimental Botany, 53:1305-1319.
doi: 10.1093/jexbot/53.372.1305 URL |
| [58] |
Silveira N M, Marcos F C C, Frungillo L, Moura B B, Seabra A B, Salgado I, Machado E C, Hancock J T, Ribeiro R V. 2017. S-nitrosoglutathione spraying improves stomatal conductance,Rubisco activity and antioxidant defense in both leaves and roots of sugarcane plants under water deficit. Physiologia Plantarum, 160:383-395.
doi: 10.1111/ppl.2017.160.issue-4 URL |
| [59] |
Singh R, Parihar P, Prasad S. 2020. Sulphur and calcium attenuate arsenic toxicity in Brassica by adjusting ascorbate-glutathione cycle and sulphur metabolism. Plant Growth Regulation, 91:221-235.
doi: 10.1007/s10725-020-00601-8 URL |
| [60] |
Spoel S, Koornneef A, Claessens S, Korzelius J, Van Pelt J, Mueller M, Buchala A, Metraux J P, Brown R, Kazan K, Loon L, Dong X, Pieterse C. 2003. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. The Plant cell, 15:760-770.
doi: 10.1105/tpc.009159 URL |
| [61] |
Srivastava M K, Dwivedi U N. 1998. Salicylic acid modulates glutathione metabolism in pea seedlings. Journal of Plant Physiology, 153:409-414.
doi: 10.1016/S0176-1617(98)80168-5 URL |
| [62] |
Stasolla C. 2010. Glutathione redox regulation of in vitro embryogenesis. Plant Physiology and Biochemistry, 48:319-327.
doi: 10.1016/j.plaphy.2009.10.007 URL |
| [63] |
Stasolla C, Belmonte M, Van Zyl L, Craig D, Liu W, Yeung E, Sederoff R. 2004. The effect of reduced glutathione on morphology and gene expression of white spruce( Picea glauca)somatic embryos. Journal of Experimental Botany, 55:695-709.
doi: 10.1093/jxb/erh074 URL |
| [64] |
Stasolla C, Belmonte M F, Tahir M, Elhiti M, Khamiss K, Joosen R, Maliepaard C, Sharpe A, Gjetvaj B, Boutilier K. 2008. Buthionine sulfoximine(BSO)-mediated improvement in cultured embryo quality in vitro entails changes in ascorbate metabolism,meristem development and embryo maturation. Planta, 228:255-272.
doi: 10.1007/s00425-008-0735-z URL pmid: 18458948 |
| [65] |
Sugimoto M, Oono Y, Gusev O, Matsumoto T, Yazawa T, Levinskikh M A, Sychev V N, Bingham G E, Wheeler R, Hummerick M. 2014. Genome-wide expression analysis of reactive oxygen species gene network in Mizuna plants grown in long-term spaceflight. BMC Plant Biology, 14:4.
doi: 10.1186/1471-2229-14-4 pmid: 24393219 |
| [66] | Sultana A, Boro P, Mandal K, Chattopadhyay S. 2020. AAL-toxin induced stress in Arabidopsis thaliana is alleviated through GSH-mediated salicylic acid and ethylene pathways. Plant Cell,Tissue and Organ Culture(PCTOC), 141:299-314. |
| [67] |
Sun C, Liu L, Yu Y, Liu W, Lu L, Jin C, Lin X. 2015. Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. Journal of Integrative Plant Biology, 57:550-561.
doi: 10.1111/jipb.12298 URL |
| [68] |
Wang C Y. 1995. Temperature preconditioning affects glutathione contentand glutathione reductase activity in chilled Zucchini squash. Journal of Plant Physiology, 145:148-152.
doi: 10.1016/S0176-1617(11)81862-6 URL |
| [69] |
Wang D, Liu Y, Tan X, Liu H, Zeng G, Hu X, Jian H, Gu Y. 2015. Effect of exogenous nitric oxide on antioxidative system and S-nitrosylation in leaves of Boehmeria nivea(L.)Gaud under cadmium stress. Environmental Science and Pollution Research International, 22:3489-3497.
doi: 10.1007/s11356-014-3581-5 URL |
| [70] | Wei L, Lina W, Yang Y, Pengfei W, Tiancai G, Guozhang K. 2015. Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Frontiers in Plant Science, 6:458. |
| [71] | Wei Li-ting. 2015. The molecular mechanism of abscisic acid enhances contents of ascorbate,glutathione and starch in wheat seedlings under polyethylene glycol-stimulated drought stress[M. D. Dissertation]. Zhengzhou:Henan Agricultural University. (in Chinese) |
| 魏利婷. 2015. 提高干旱胁迫小麦幼苗抗坏血酸、谷胱甘肽和淀粉含量的分子机理[硕士论文]. 郑州:河南农业大学. | |
| [72] | Wongkaew A, Asayama K, Kitaiwa T, Nakamura S I, Kojima K, Stacey G, Sekimoto H, Yokoyama T, Ohkama-Ohtsu N. 2018. AtOPT 6 protein functions in long-distance transport of glutathione in Arabidopsis thaliana. Plant and Cell Physiology, 59:1443-1451. |
| [73] |
Wu Y, Hu L, Liao W, Mujitaba Dawuda M, Lyu J, Xie J, Feng Z, Calderón-Urrea A, Yu J. 2019. Foliar application of 5-aminolevulinic acid(ALA)alleviates NaCl stress in cucumber( Cucumis sativus L.)seedlings through the enhancement of ascorbate-glutathione cycle. Scientia Horticulturae, 257:108761.
doi: 10.1016/j.scienta.2019.108761 URL |
| [74] |
Yamazaki S, Ochiai K, Matoh T. 2019. Rice plants have three homologs of glutathione synthetase genes,one of which, OsGS2,codes for hydroxymethyl-glutathione synthetase. Plant Direct, 3:e00119.
doi: 10.1002/pld3.119 URL |
| [75] |
Yang J, Gao M X, Hu H, Ding X M, Lin H W, Wang L, Xu J M, Mao C Z, Zhao F J, Wu Z C. 2016. OsCLT1,a CRT-like transporter 1,is required for glutathione homeostasis and arsenic tolerance in rice. New Phytologist, 211:658-670.
doi: 10.1111/nph.2016.211.issue-2 URL |
| [76] |
Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Zhang J, Theprungsirikul L, Shrift T, Krichilsky B, Johnson D M, Swift G B, He Y, Siedow J N, Pei Z M. 2014. OSCA 1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature, 514:367-371.
doi: 10.1038/nature13593 URL |
| [77] |
Yuan L B, Dai Y S, Xie L J, Yu L J, Zhou Y, Lai Y X, Yang Y C, Xu L, Chen Q F, Xiao S. 2017. Jasmonate regulates plant responses to postsubmergence reoxygenation through transcriptional activation of antioxidant synthesis. Plant Physiology, 173:1864-1880.
doi: 10.1104/pp.16.01803 URL |
| [78] | Zeng X, Qiu D, Hu R, Zhang M. Glutathione transporters in plants//Hossain M A, Mostofa M G, Diaz-Vivancos P. 2017. Glutathione in plant growth,development,and stress tolerance. Cham: Springer International Publishing. |
| [79] |
Zhan N, Wang C, Chen L, Yang H, Feng J, Gong X, Ren B, Wu R, Mu J, Li Y, Liu Z, Zhou Y, Peng J, Wang K, Huang X, Xiao S, Zuo J. 2018. S-Nitrosylation targets GSNO reductase for selective autophagy during hypoxia responses in plants. Molecular Cell, 71:142-154.
doi: S1097-2765(18)30398-8 pmid: 30008318 |
| [80] |
Zhang Teng-guo, Nie Ting-ting, Sun Wan-cang, Shi Zhong-fei, Wang Juan. 2018. Effects of diverse stresses on gene expression and enzyme activity of glutathione reductase in Brassica campestris. Chinese Journal of Applied Ecology, 29 (1):213-222. (in Chinese)
doi: 10.13287/j.1001-9332.201801.010 pmid: 29692030 |
|
张腾国, 聂亭亭, 孙万仓, 史中飞, 王娟. 2018. 逆境胁迫对油菜谷胱甘肽还原酶基因表达及其酶活性的影响. 应用生态学报, 29 (1):213-222.
pmid: 29692030 |
|
| [81] |
Zhang Z, Xie Q, Jobe T O, Kau A R, Wang C, Li Y, Qiu B, Wang Q, Mendoza-Cózatl D G, Schroeder J I. 2016. Identification of AtOPT4 as a Plant Glutathione Transporter. Molecular Plant, 9:481-484.
doi: 10.1016/j.molp.2015.07.013 URL |
| [82] |
Zhu J K. 2016. Abiotic stress signaling and responses in plants. Cell, 167:313-324.
doi: 10.1016/j.cell.2016.08.029 URL |
| [1] | 徐小萍, 曹清影, 蔡柔荻, 官庆栩, 张梓浩, 陈裕坤, 徐涵, 林玉玲, 赖钟雄. 龙眼miR408与DlLAC12克隆及其在球形胚发生和非生物胁迫下的表达分析[J]. 园艺学报, 2022, 49(9): 1866-1882. |
| [2] | 贾鑫, 曾臻, 陈月, 冯慧, 吕英民, 赵世伟. 月季‘月月粉’RcDREB2A的克隆与表达分析[J]. 园艺学报, 2022, 49(9): 1945-1956. |
| [3] | 马维峰, 李艳梅, 马宗桓, 陈佰鸿, 毛娟. 苹果POD家族基因的鉴定与MdPOD15的功能分析[J]. 园艺学报, 2022, 49(6): 1181-1199. |
| [4] | 李丽仙, 王烁, 陈莹, 邬滢涛, 王雅倩, 房月, 陈学森, 田长平, 冯守千. 甜樱桃PavMYB10.1促进PavRiant表达和花青苷积累[J]. 园艺学报, 2022, 49(5): 1023-1030. |
| [5] | 周至铭, 杨佳宝, 张程, 曾令露, 孟晚秋, 孙黎. 向日葵LACS家族鉴定及响应非生物胁迫表达分析[J]. 园艺学报, 2022, 49(2): 352-364. |
| [6] | 谢思艺, 周承哲, 朱晨, 詹冬梅, 陈兰, 吴祖春, 赖钟雄, 郭玉琼. 茶树CsTIFY家族全基因组鉴定及非生物胁迫和激素处理中主要基因表达分析[J]. 园艺学报, 2022, 49(1): 100-116. |
| [7] | 梁志乐, 汪宽鸿, 杨静, 祝彪, 朱祝军. 硫代葡萄糖苷在十字花科植物应对非生物胁迫中的作用[J]. 园艺学报, 2022, 49(1): 200-220. |
| [8] | 杨天宸, 陈晓童, 吕可, 张荻. 百子莲脱水素基因ApSK3对逆境与激素信号的应答模式与调控机制[J]. 园艺学报, 2021, 48(8): 1565-1578. |
| [9] | 马俊杰, 宋丽娜, 李乐, 马晓春, 靳磊, 徐伟荣. 山葡萄VaCBL6参与非生物胁迫和ABA途径的响应[J]. 园艺学报, 2021, 48(6): 1079-1093. |
| [10] | 岳玲琦, 邢巧娟, 张晓兰, 梁雪, 王乾, 齐红岩. 光敏色素互作因子在植物抵御逆境胁迫中的作用研究进展[J]. 园艺学报, 2021, 48(4): 632-646. |
| [11] | 白露, 张志国, 张世杰, 黄东梅, 秦巧平. 萱草3种蔗糖转化酶基因的分离及对低温和渗透胁迫响应的分析[J]. 园艺学报, 2021, 48(2): 300-312. |
| [12] | 张婷婷, 李雨欣, 张德遥, 康宇乾, 王健, 宋希强, 周扬. 铁皮石斛蛋白磷酸酶PP2C家族基因鉴定及其表达分析[J]. 园艺学报, 2021, 48(12): 2458-2470. |
| [13] | 柯玉洁, 陈明堃, 马山虎, 欧悦, 王艺, 郑清冬, 刘仲健, 艾叶. 兰科植物MYB转录因子研究进展[J]. 园艺学报, 2021, 48(11): 2311-2320. |
| [14] | 李欣欣, 侯鸿敏, 徐吉花, 孙晓红, 张玉刚. 苹果MLP家族基因鉴定及非生物胁迫响应分析[J]. 园艺学报, 2021, 48(1): 1-14. |
| [15] | 葛诗蓓, 姜小春, 王羚羽, 喻景权, 周艳虹. 园艺植物丛枝菌根抗非生物胁迫的作用机制研究进展[J]. 园艺学报, 2020, 47(9): 1752-1776. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司