
园艺学报 ›› 2023, Vol. 50 ›› Issue (4): 737-753.doi: 10.16420/j.issn.0513-353x.2022-0044
李可1, 申朦晓1, 潘炜浩1, 章诗渲1, 毛欣烨1, 尹亚红1, 李永强1,2,*( ), 朱友银3,*(
), 朱友银3,*( ), 郭卫东1,2
), 郭卫东1,2
收稿日期:2022-10-08
修回日期:2023-01-21
出版日期:2023-04-25
发布日期:2023-04-27
通讯作者:
*(E-mail:lyq@zjnu.cn,基金资助:
LI Ke1, SHEN Mengxiao1, PAN Weihao1, ZHANG Shixuan1, MAO Xinye1, YIN Yahong1, LI Yongqiang1,2,*( ), ZHU Youyin3,*(
), ZHU Youyin3,*( ), GUO Weidong1,2
), GUO Weidong1,2
Received:2022-10-08
Revised:2023-01-21
Online:2023-04-25
Published:2023-04-27
Contact:
*(E-mail:lyq@zjnu.cn,zhuyouyin@jhc.edu.cn)
E-mail:lyq@zjnu.cn;zhuyouyin@jhc.edu.cn
摘要:
为了解蓝莓C2H2锌指蛋白在花芽休眠中的功能,通过生物信息鉴定蓝莓C2H2-ZFP基因家族成员,利用转录组数据和荧光定量PCR分析其在花芽内休眠解除过程和外源脱落酸处理下的表达模式。在蓝莓中共鉴定到VcC2H2-ZFP家族79个成员,通过系统进化树和保守基序将其分为3组。分析表明:VcC2H2-ZFP家族成员在蓝莓各器官中均有表达,其中15个在花芽内休眠解除过程中表达存在显著差异,如Vc31g303.5、Vc7g51.6、Vc16g355.5在深度内休眠时期表达量高,而休眠解除后表达量较低。VcC2H2-ZFP启动子含有大量光反应、防御应激、脱落酸、赤霉素等激素响应元件。外源脱落酸处理花芽能显著促进Vc16g355.5、Vc7g51.6、Vc31g303.5等的表达。本研究结果揭示蓝莓部分VcC2H2-ZFP可能通过响应脱落酸参与花芽休眠过程的调控。
中图分类号:
李可, 申朦晓, 潘炜浩, 章诗渲, 毛欣烨, 尹亚红, 李永强, 朱友银, 郭卫东. 蓝莓C2H2家族基因在花芽休眠解除中的作用初探[J]. 园艺学报, 2023, 50(4): 737-753.
LI Ke, SHEN Mengxiao, PAN Weihao, ZHANG Shixuan, MAO Xinye, YIN Yahong, LI Yongqiang, ZHU Youyin, GUO Weidong. Preliminary Investigation of C2H2 Family Genes in Blueberry Flower Bud Dormancy Release[J]. Acta Horticulturae Sinica, 2023, 50(4): 737-753.
| 目的基因 Gene name | 上游引物(5′-3′) Forward primer | 下游引物(5′-3′) Reverse primer | 扩增长度/bp Amplicon size | 退火温度/℃ Tm |
|---|---|---|---|---|
| Vc38g176.32 | TTATTCGGTGGTTCACGGCA | TTGCGAAGTCACGGATGGAA | 365 | 55 |
| Vc7g51.6 | GCTCGATCTGTCACAAGAGTT | GACGTCGTAAGTCCACTGTTT | 109 | 55 |
| Vc3g10.6 | AACAGAGGATTCGACCTGAAC | CCCAAATCAGGTTGGAAAGATAAA | 155 | 55 |
| Vc31g303.5 | GGAGGAGCAAACAGTGGAATTA | CAAATCCGAATTCCGGCAAAG | 112 | 55 |
| Vc24g106.5 | AAGAAGACGAAGAAGGAGGTAATAAG | GCGACACCGATTTGGTAGAA | 192 | 55 |
| Vc16g355.5 | GGAGGAGCAAACAGTGGAATTA | CCAAATCCATATTCCGGCAAAG | 113 | 55 |
| Vc14g348.9 | GTGTGACGACGACATCTGAA | CCCAAATCAGGTTGGAAAGATAAA | 196 | 55 |
| Vc11g294.5 | CGATATGTGGGTCGGAGTTT | CATCGGTGCTGGTAGATTCA | 183 | 55 |
| Vc21g155.6 | CAAAGGGCCCAACTGCAACAGAT | TACACCCCGAGAAGGGACACGAA | 483 | 55 |
| Vc23g300.2 | CCACAGGTGCTTTCCAACGGGTCA | CGCCGAATCCTTGCCAGAATTCAG | 194 | 60.5 |
| Vc37g104.16 | CTGATAAAATGGAGACTGATGAAA | CCAGACAACAGATAAGAAAACGAA | 244 | 60.5 |
| Vc37g230.20 | TCGACAAAGGTGGTTCAGGT | TGTCCCTGTACCCATACCCA | 213 | 60.5 |
| Vc38g80.29 | GAAGAGGAGAAAGGGAATGCCACT | AACCCATCAAGCAATCCATCAAAA | 152 | 60.5 |
| Vc42g36.8 | GCAAAGGGCCCAACTGCAACAGAT | CACCCCGAGAAGGGACACGAAAAC | 482 | 60.5 |
| Vc6g338.31 | GAAGAGGAGAAAGGGAATGCCACT | AACCCATCAAGCAATCCATCAAAA | 152 | 62 |
| VcGAPDH | TGAGAAAGAATACAAGCCAGAT | CAGGCAACACCTTACCAA | 260 | 55 ~ 62 |
表1 VcC2H2-ZFP定量引物
Table 1 Quantitative primers of VcC2H2-ZFP
| 目的基因 Gene name | 上游引物(5′-3′) Forward primer | 下游引物(5′-3′) Reverse primer | 扩增长度/bp Amplicon size | 退火温度/℃ Tm |
|---|---|---|---|---|
| Vc38g176.32 | TTATTCGGTGGTTCACGGCA | TTGCGAAGTCACGGATGGAA | 365 | 55 |
| Vc7g51.6 | GCTCGATCTGTCACAAGAGTT | GACGTCGTAAGTCCACTGTTT | 109 | 55 |
| Vc3g10.6 | AACAGAGGATTCGACCTGAAC | CCCAAATCAGGTTGGAAAGATAAA | 155 | 55 |
| Vc31g303.5 | GGAGGAGCAAACAGTGGAATTA | CAAATCCGAATTCCGGCAAAG | 112 | 55 |
| Vc24g106.5 | AAGAAGACGAAGAAGGAGGTAATAAG | GCGACACCGATTTGGTAGAA | 192 | 55 |
| Vc16g355.5 | GGAGGAGCAAACAGTGGAATTA | CCAAATCCATATTCCGGCAAAG | 113 | 55 |
| Vc14g348.9 | GTGTGACGACGACATCTGAA | CCCAAATCAGGTTGGAAAGATAAA | 196 | 55 |
| Vc11g294.5 | CGATATGTGGGTCGGAGTTT | CATCGGTGCTGGTAGATTCA | 183 | 55 |
| Vc21g155.6 | CAAAGGGCCCAACTGCAACAGAT | TACACCCCGAGAAGGGACACGAA | 483 | 55 |
| Vc23g300.2 | CCACAGGTGCTTTCCAACGGGTCA | CGCCGAATCCTTGCCAGAATTCAG | 194 | 60.5 |
| Vc37g104.16 | CTGATAAAATGGAGACTGATGAAA | CCAGACAACAGATAAGAAAACGAA | 244 | 60.5 |
| Vc37g230.20 | TCGACAAAGGTGGTTCAGGT | TGTCCCTGTACCCATACCCA | 213 | 60.5 |
| Vc38g80.29 | GAAGAGGAGAAAGGGAATGCCACT | AACCCATCAAGCAATCCATCAAAA | 152 | 60.5 |
| Vc42g36.8 | GCAAAGGGCCCAACTGCAACAGAT | CACCCCGAGAAGGGACACGAAAAC | 482 | 60.5 |
| Vc6g338.31 | GAAGAGGAGAAAGGGAATGCCACT | AACCCATCAAGCAATCCATCAAAA | 152 | 62 |
| VcGAPDH | TGAGAAAGAATACAAGCCAGAT | CAGGCAACACCTTACCAA | 260 | 55 ~ 62 |
| 基因名 Gene name | 基因ID Accession No. | 氨基酸数 Amino acid number | 分子量/kD Molecular weight | 等电点 pI | 不稳定系数 Instability index | 亲水值 Hydropathicity | 亚细胞定位 Subcellular location |
|---|---|---|---|---|---|---|---|
| Vc19g325.6 | VaccDscaff19-processed-325.6 | 396 | 43.8 | 8.94 | 49.42 | -0.696 | Nucl,Cyto |
| Vc15g278.5 | VaccDscaff15-processed-278.5 | 394 | 43.7 | 9.00 | 46.09 | -0.704 | Nucl,Cyto |
| Vc11g294.4 | VaccDscaff11-processed-294.4 | 398 | 44.2 | 9.01 | 48.83 | -0.727 | Nucl,Cyto |
| Vc24g106.6 | VaccDscaff24-processed-106.6 | 398 | 44.2 | 8.94 | 48.52 | -0.734 | Nucl,Cyto |
| Vc30g269.43 | VaccDscaff30-augustus-269.43 | 508 | 56.4 | 6.79 | 41.71 | -0.436 | Nucl,Chlo |
| Vc13g13.36 | VaccDscaff13-augustus-13.36 | 456 | 50.7 | 5.87 | 41.61 | -0.398 | Nucl |
| Vc42g47.29 | VaccDscaff42-augustus-47.29 | 456 | 50.6 | 5.87 | 41.10 | -0.397 | Nucl |
| Vc47g65.34 | VaccDscaff47-augustus-65.34 | 388 | 42.9 | 8.51 | 45.21 | -0.701 | Nucl |
| Vc43g136.13 | VaccDscaff43-augustus-136.13 | 379 | 41.9 | 8.61 | 46.09 | -0.635 | Nucl |
| Vc22g249.38 | VaccDscaff22-augustus-249.38 | 378 | 41.7 | 8.61 | 43.32 | -0.599 | Nucl |
| Vc39g85.33 | VaccDscaff39-augustus-85.33 | 381 | 42.3 | 5.60 | 44.46 | -0.452 | Nucl |
| Vc37g230.20 | VaccDscaff37-augustus-230.20 | 392 | 43.3 | 5.58 | 42.06 | -0.421 | Nucl |
| Vc38g80.29 | VaccDscaff38-snap-80.29 | 392 | 43.1 | 5.44 | 43.73 | -0.430 | Nucl |
| Vc6g338.31 | VaccDscaff6-augustus-338.31 | 392 | 43.2 | 5.43 | 42.87 | -0.438 | Nucl,Pero |
| Vc38g176.32 | VaccDscaff38-augustus-176.32 | 478 | 53.4 | 5.84 | 50.44 | -0.775 | Nucl,Pero |
| Vc6g193.34 | VaccDscaff6-augustus-193.34 | 478 | 53.4 | 5.84 | 50.44 | -0.775 | Nucl,Pero |
| Vc37g104.16 | VaccDscaff37-augustus-104.16 | 478 | 53.4 | 5.84 | 50.88 | -0.756 | Nucl,Pero |
| Vc32g62.9 | VaccDscaff32-processed-62.9 | 382 | 41.4 | 6.57 | 53.67 | -0.656 | Nucl |
| Vc32g14.2 | VaccDscaff32-processed-14.2 | 474 | 52.0 | 8.39 | 54.84 | -0.510 | Nucl |
| Vc13g317.8 | VaccDscaff13-processed-317.8 | 477 | 52.1 | 8.09 | 55.80 | -0.521 | Nucl |
| Vc30g79.13 | VaccDscaff30-processed-79.13 | 474 | 52.0 | 8.07 | 54.80 | -0.524 | Nucl |
| Vc19g325.7 | VaccDscaff19-processed-325.7 | 254 | 27.9 | 5.94 | 64.39 | -0.774 | Nucl |
| Vc15g278.6 | VaccDscaff15-processed-278.6 | 254 | 27.9 | 5.94 | 64.05 | -0.784 | Nucl |
| Vc11g294.5 | VaccDscaff11-processed-294.5 | 254 | 28.0 | 5.81 | 65.34 | -0.805 | Nucl |
| Vc24g106.5 | VaccDscaff24-processed-106.5 | 254 | 28.0 | 5.88 | 63.53 | -0.826 | Nucl |
| Vc33g157.2 | VaccDscaff33-processed-157.2 | 244 | 27.5 | 7.11 | 74.79 | -0.976 | Nucl |
| Vc21g197.4 | VaccDscaff21-processed-197.4 | 245 | 27.6 | 7.11 | 73.79 | -0.938 | Nucl |
| Vc29g187.1 | VaccDscaff29-processed-187.1 | 245 | 27.6 | 7.11 | 74.52 | -0.953 | Nucl |
| Vc19g69.12 | VaccDscaff19-processed-69.12 | 355 | 39.0 | 5.51 | 69.83 | -0.699 | Nucl |
| Vc26g194.1 | VaccDscaff26-processed-194.1 | 244 | 27.5 | 6.51 | 76.04 | -0.933 | Nucl |
| Vc20g74.12 | VaccDscaff20-processed-74.12 | 355 | 38.8 | 5.51 | 68.93 | -0.683 | Nucl |
| Vc28g285.19 | VaccDscaff28-processed-285.19 | 355 | 38.9 | 5.49 | 68.69 | -0.692 | Nucl |
| Vc21g236.10 | VaccDscaff21-processed-236.10 | 286 | 31.0 | 7.65 | 56.50 | -0.810 | Nucl |
| Vc26g232.8 | VaccDscaff26-processed-232.8 | 286 | 31.1 | 8.14 | 54.76 | -0.819 | Nucl |
| Vc33g119.6 | VaccDscaff33-processed-119.6 | 287 | 31.1 | 7.65 | 54.81 | -0.808 | Nucl |
| Vc40g163.1 | VaccDscaff40-processed-163.1 | 371 | 41.9 | 5.75 | 64.69 | -1.037 | Nucl |
| Vc41g152.1 | VaccDscaff41-processed-152.1 | 373 | 42.1 | 5.82 | 62.04 | -1.050 | Nucl |
| Vc23g116.9 | VaccDscaff23-processed-116.9 | 373 | 42.1 | 5.68 | 61.92 | -1.053 | Nucl |
| Vc23g130.8 | VaccDscaff23-processed-130.8 | 371 | 41.8 | 5.81 | 64.61 | -1.011 | Nucl |
| Vc29g224.1 | VaccDscaff29-processed-224.1 | 275 | 29.7 | 8.45 | 53.45 | -0.817 | Nucl,Extr |
| Vc47g65.8 | VaccDscaff47-processed-65.8 | 275 | 29.9 | 6.32 | 56.61 | -0.635 | Nucl |
| Vc18g40.7 | VaccDscaff18-processed-40.7 | 236 | 24.6 | 8.93 | 59.17 | -0.567 | Nucl |
| Vc43g135.1 | VaccDscaff43-processed-135.1 | 272 | 29.6 | 6.25 | 55.65 | -0.584 | Nucl |
| Vc16g355.5 | VaccDscaff16-processed-355.5 | 234 | 24.4 | 8.92 | 59.76 | -0.562 | Nucl |
| Vc22g250.7 | VaccDscaff22-processed-250.7 | 272 | 29.6 | 6.25 | 55.53 | -0.606 | Nucl |
| Vc7g51.6 | VaccDscaff7-processed-51.6 | 224 | 23.5 | 9.01 | 62.98 | -0.544 | Nucl |
| Vc31g303.5 | VaccDscaff31-processed-303.5 | 233 | 24.3 | 8.93 | 61.63 | -0.561 | Nucl |
| Vc40g40.5 | VaccDscaff40-processed-40.5 | 240 | 25.5 | 7.72 | 71.73 | -0.707 | Nucl |
| Vc23g300.2 | VaccDscaff23-processed-300.2 | 239 | 25.4 | 8.22 | 71.35 | -0.700 | Nucl |
| Vc12g112.2 | VaccDscaff12-processed-112.2 | 240 | 25.5 | 8.17 | 70.40 | -0.668 | Nucl |
| Vc1g306.32 | VaccDscaff1-processed-306.32 | 239 | 25.8 | 7.87 | 57.87 | -0.782 | Nucl |
| Vc10g124.14 | VaccDscaff10-processed-124.14 | 306 | 33.2 | 7.86 | 64.71 | -0.660 | Nucl |
| Vc3g10.6 | VaccDscaff3-processed-10.6 | 217 | 23.4 | 8.68 | 67.52 | -0.675 | Nucl |
| Vc14g348.9 | VaccDscaff14-processed-348.9 | 233 | 24.7 | 8.67 | 61.00 | -0.624 | Nucl |
| Vc2g402.7 | VaccDscaff2-processed-402.7 | 234 | 24.8 | 8.67 | 59.96 | -0.573 | Nucl |
| Vc22g45.12 | VaccDscaff22-processed-45.12 | 371 | 42.3 | 6.26 | 58.05 | -1.124 | Nucl |
| Vc21g155.6 | VaccDscaff21-processed-155.6 | 182 | 20.4 | 8.84 | 51.53 | -0.348 | Nucl |
| Vc26g158.3 | VaccDscaff26-processed-158.3 | 182 | 20.4 | 8.67 | 52.00 | -0.348 | Nucl |
| Vc33g183.0 | VaccDscaff33-processed-183.0 | 182 | 20.4 | 8.67 | 52.00 | -0.348 | Nucl |
| Vc29g155.8 | VaccDscaff29-processed-155.8 | 182 | 20.4 | 8.66 | 55.59 | -0.415 | Nucl |
| Vc20g57.10 | VaccDscaff20-processed-57.10 | 161 | 17.7 | 9.02 | 53.48 | -0.437 | Nucl,Cyto,Extr |
| Vc48g64.13 | VaccDscaff48-processed-64.13 | 173 | 18.9 | 9.34 | 48.58 | -0.395 | Nucl,Cyto,Extr |
| Vc14g51.11 | VaccDscaff14-processed-51.11 | 228 | 25.1 | 5.19 | 64.33 | -0.483 | Nucl,Pero |
| Vc20g133.0 | VaccDscaff20-processed-133.0 | 228 | 24.2 | 7.08 | 60.52 | -0.638 | Nucl |
| Vc28g213.2 | VaccDscaff28-processed-213.2 | 228 | 24.2 | 7.08 | 60.52 | -0.638 | Nucl |
| Vc2g14.22 | VaccDscaff2-processed-14.22 | 228 | 25.2 | 5.07 | 61.95 | -0.466 | Nucl,Pero |
| Vc42g36.8 | VaccDscaff42-processed-36.8 | 232 | 25.4 | 5.16 | 46.05 | -0.316 | Nucl,Pero |
| Vc19g135.9 | VaccDscaff19-processed-135.9 | 222 | 23.7 | 7.08 | 55.23 | -0.605 | Nucl,Pero |
| Vc30g280.2 | VaccDscaff30-processed-280.2 | 232 | 25.5 | 5.16 | 46.41 | -0.319 | Nucl |
| Vc27g295.0 | VaccDscaff27-processed-295.0 | 263 | 28.8 | 8.80 | 62.35 | -0.655 | Nucl,Pero |
| Vc17g324.14 | VaccDscaff17-processed-324.14 | 263 | 28.8 | 8.59 | 62.70 | -0.664 | Nucl,Pero |
| Vc34g49.12 | VaccDscaff34-processed-49.12 | 263 | 28.8 | 8.59 | 62.70 | -0.664 | Nucl,Pero |
| Vc44g170.19 | VaccDscaff44-augustus-170.19 | 248 | 27.0 | 7.14 | 60.50 | -0.597 | Nucl |
| Vc20g156.15 | VaccDscaff20-augustus-156.15 | 248 | 27.0 | 6.50 | 57.33 | -0.583 | Nucl |
| Vc28g167.32 | VaccDscaff28-augustus-167.32 | 248 | 27.0 | 6.75 | 60.99 | -0.583 | Nucl |
| Vc44g65.1 | VaccDscaff44-processed-65.1 | 247 | 26.6 | 8.38 | 54.41 | -0.681 | Nucl |
| Vc28g73.2 | VaccDscaff28-processed-73.2 | 247 | 26.6 | 8.38 | 53.87 | -0.681 | Nucl |
| Vc20g302.3 | VaccDscaff20-processed-302.3 | 250 | 26.9 | 7.80 | 55.74 | -0.688 | Nucl |
| Vc8g145.9 | VaccDscaff8-processed-145.9 | 237 | 25.8 | 7.87 | 57.57 | -0.784 | Nucl |
表2 VcC2H2-ZFP基本信息
Table 2 Basic information of VcC2H2-ZFP
| 基因名 Gene name | 基因ID Accession No. | 氨基酸数 Amino acid number | 分子量/kD Molecular weight | 等电点 pI | 不稳定系数 Instability index | 亲水值 Hydropathicity | 亚细胞定位 Subcellular location |
|---|---|---|---|---|---|---|---|
| Vc19g325.6 | VaccDscaff19-processed-325.6 | 396 | 43.8 | 8.94 | 49.42 | -0.696 | Nucl,Cyto |
| Vc15g278.5 | VaccDscaff15-processed-278.5 | 394 | 43.7 | 9.00 | 46.09 | -0.704 | Nucl,Cyto |
| Vc11g294.4 | VaccDscaff11-processed-294.4 | 398 | 44.2 | 9.01 | 48.83 | -0.727 | Nucl,Cyto |
| Vc24g106.6 | VaccDscaff24-processed-106.6 | 398 | 44.2 | 8.94 | 48.52 | -0.734 | Nucl,Cyto |
| Vc30g269.43 | VaccDscaff30-augustus-269.43 | 508 | 56.4 | 6.79 | 41.71 | -0.436 | Nucl,Chlo |
| Vc13g13.36 | VaccDscaff13-augustus-13.36 | 456 | 50.7 | 5.87 | 41.61 | -0.398 | Nucl |
| Vc42g47.29 | VaccDscaff42-augustus-47.29 | 456 | 50.6 | 5.87 | 41.10 | -0.397 | Nucl |
| Vc47g65.34 | VaccDscaff47-augustus-65.34 | 388 | 42.9 | 8.51 | 45.21 | -0.701 | Nucl |
| Vc43g136.13 | VaccDscaff43-augustus-136.13 | 379 | 41.9 | 8.61 | 46.09 | -0.635 | Nucl |
| Vc22g249.38 | VaccDscaff22-augustus-249.38 | 378 | 41.7 | 8.61 | 43.32 | -0.599 | Nucl |
| Vc39g85.33 | VaccDscaff39-augustus-85.33 | 381 | 42.3 | 5.60 | 44.46 | -0.452 | Nucl |
| Vc37g230.20 | VaccDscaff37-augustus-230.20 | 392 | 43.3 | 5.58 | 42.06 | -0.421 | Nucl |
| Vc38g80.29 | VaccDscaff38-snap-80.29 | 392 | 43.1 | 5.44 | 43.73 | -0.430 | Nucl |
| Vc6g338.31 | VaccDscaff6-augustus-338.31 | 392 | 43.2 | 5.43 | 42.87 | -0.438 | Nucl,Pero |
| Vc38g176.32 | VaccDscaff38-augustus-176.32 | 478 | 53.4 | 5.84 | 50.44 | -0.775 | Nucl,Pero |
| Vc6g193.34 | VaccDscaff6-augustus-193.34 | 478 | 53.4 | 5.84 | 50.44 | -0.775 | Nucl,Pero |
| Vc37g104.16 | VaccDscaff37-augustus-104.16 | 478 | 53.4 | 5.84 | 50.88 | -0.756 | Nucl,Pero |
| Vc32g62.9 | VaccDscaff32-processed-62.9 | 382 | 41.4 | 6.57 | 53.67 | -0.656 | Nucl |
| Vc32g14.2 | VaccDscaff32-processed-14.2 | 474 | 52.0 | 8.39 | 54.84 | -0.510 | Nucl |
| Vc13g317.8 | VaccDscaff13-processed-317.8 | 477 | 52.1 | 8.09 | 55.80 | -0.521 | Nucl |
| Vc30g79.13 | VaccDscaff30-processed-79.13 | 474 | 52.0 | 8.07 | 54.80 | -0.524 | Nucl |
| Vc19g325.7 | VaccDscaff19-processed-325.7 | 254 | 27.9 | 5.94 | 64.39 | -0.774 | Nucl |
| Vc15g278.6 | VaccDscaff15-processed-278.6 | 254 | 27.9 | 5.94 | 64.05 | -0.784 | Nucl |
| Vc11g294.5 | VaccDscaff11-processed-294.5 | 254 | 28.0 | 5.81 | 65.34 | -0.805 | Nucl |
| Vc24g106.5 | VaccDscaff24-processed-106.5 | 254 | 28.0 | 5.88 | 63.53 | -0.826 | Nucl |
| Vc33g157.2 | VaccDscaff33-processed-157.2 | 244 | 27.5 | 7.11 | 74.79 | -0.976 | Nucl |
| Vc21g197.4 | VaccDscaff21-processed-197.4 | 245 | 27.6 | 7.11 | 73.79 | -0.938 | Nucl |
| Vc29g187.1 | VaccDscaff29-processed-187.1 | 245 | 27.6 | 7.11 | 74.52 | -0.953 | Nucl |
| Vc19g69.12 | VaccDscaff19-processed-69.12 | 355 | 39.0 | 5.51 | 69.83 | -0.699 | Nucl |
| Vc26g194.1 | VaccDscaff26-processed-194.1 | 244 | 27.5 | 6.51 | 76.04 | -0.933 | Nucl |
| Vc20g74.12 | VaccDscaff20-processed-74.12 | 355 | 38.8 | 5.51 | 68.93 | -0.683 | Nucl |
| Vc28g285.19 | VaccDscaff28-processed-285.19 | 355 | 38.9 | 5.49 | 68.69 | -0.692 | Nucl |
| Vc21g236.10 | VaccDscaff21-processed-236.10 | 286 | 31.0 | 7.65 | 56.50 | -0.810 | Nucl |
| Vc26g232.8 | VaccDscaff26-processed-232.8 | 286 | 31.1 | 8.14 | 54.76 | -0.819 | Nucl |
| Vc33g119.6 | VaccDscaff33-processed-119.6 | 287 | 31.1 | 7.65 | 54.81 | -0.808 | Nucl |
| Vc40g163.1 | VaccDscaff40-processed-163.1 | 371 | 41.9 | 5.75 | 64.69 | -1.037 | Nucl |
| Vc41g152.1 | VaccDscaff41-processed-152.1 | 373 | 42.1 | 5.82 | 62.04 | -1.050 | Nucl |
| Vc23g116.9 | VaccDscaff23-processed-116.9 | 373 | 42.1 | 5.68 | 61.92 | -1.053 | Nucl |
| Vc23g130.8 | VaccDscaff23-processed-130.8 | 371 | 41.8 | 5.81 | 64.61 | -1.011 | Nucl |
| Vc29g224.1 | VaccDscaff29-processed-224.1 | 275 | 29.7 | 8.45 | 53.45 | -0.817 | Nucl,Extr |
| Vc47g65.8 | VaccDscaff47-processed-65.8 | 275 | 29.9 | 6.32 | 56.61 | -0.635 | Nucl |
| Vc18g40.7 | VaccDscaff18-processed-40.7 | 236 | 24.6 | 8.93 | 59.17 | -0.567 | Nucl |
| Vc43g135.1 | VaccDscaff43-processed-135.1 | 272 | 29.6 | 6.25 | 55.65 | -0.584 | Nucl |
| Vc16g355.5 | VaccDscaff16-processed-355.5 | 234 | 24.4 | 8.92 | 59.76 | -0.562 | Nucl |
| Vc22g250.7 | VaccDscaff22-processed-250.7 | 272 | 29.6 | 6.25 | 55.53 | -0.606 | Nucl |
| Vc7g51.6 | VaccDscaff7-processed-51.6 | 224 | 23.5 | 9.01 | 62.98 | -0.544 | Nucl |
| Vc31g303.5 | VaccDscaff31-processed-303.5 | 233 | 24.3 | 8.93 | 61.63 | -0.561 | Nucl |
| Vc40g40.5 | VaccDscaff40-processed-40.5 | 240 | 25.5 | 7.72 | 71.73 | -0.707 | Nucl |
| Vc23g300.2 | VaccDscaff23-processed-300.2 | 239 | 25.4 | 8.22 | 71.35 | -0.700 | Nucl |
| Vc12g112.2 | VaccDscaff12-processed-112.2 | 240 | 25.5 | 8.17 | 70.40 | -0.668 | Nucl |
| Vc1g306.32 | VaccDscaff1-processed-306.32 | 239 | 25.8 | 7.87 | 57.87 | -0.782 | Nucl |
| Vc10g124.14 | VaccDscaff10-processed-124.14 | 306 | 33.2 | 7.86 | 64.71 | -0.660 | Nucl |
| Vc3g10.6 | VaccDscaff3-processed-10.6 | 217 | 23.4 | 8.68 | 67.52 | -0.675 | Nucl |
| Vc14g348.9 | VaccDscaff14-processed-348.9 | 233 | 24.7 | 8.67 | 61.00 | -0.624 | Nucl |
| Vc2g402.7 | VaccDscaff2-processed-402.7 | 234 | 24.8 | 8.67 | 59.96 | -0.573 | Nucl |
| Vc22g45.12 | VaccDscaff22-processed-45.12 | 371 | 42.3 | 6.26 | 58.05 | -1.124 | Nucl |
| Vc21g155.6 | VaccDscaff21-processed-155.6 | 182 | 20.4 | 8.84 | 51.53 | -0.348 | Nucl |
| Vc26g158.3 | VaccDscaff26-processed-158.3 | 182 | 20.4 | 8.67 | 52.00 | -0.348 | Nucl |
| Vc33g183.0 | VaccDscaff33-processed-183.0 | 182 | 20.4 | 8.67 | 52.00 | -0.348 | Nucl |
| Vc29g155.8 | VaccDscaff29-processed-155.8 | 182 | 20.4 | 8.66 | 55.59 | -0.415 | Nucl |
| Vc20g57.10 | VaccDscaff20-processed-57.10 | 161 | 17.7 | 9.02 | 53.48 | -0.437 | Nucl,Cyto,Extr |
| Vc48g64.13 | VaccDscaff48-processed-64.13 | 173 | 18.9 | 9.34 | 48.58 | -0.395 | Nucl,Cyto,Extr |
| Vc14g51.11 | VaccDscaff14-processed-51.11 | 228 | 25.1 | 5.19 | 64.33 | -0.483 | Nucl,Pero |
| Vc20g133.0 | VaccDscaff20-processed-133.0 | 228 | 24.2 | 7.08 | 60.52 | -0.638 | Nucl |
| Vc28g213.2 | VaccDscaff28-processed-213.2 | 228 | 24.2 | 7.08 | 60.52 | -0.638 | Nucl |
| Vc2g14.22 | VaccDscaff2-processed-14.22 | 228 | 25.2 | 5.07 | 61.95 | -0.466 | Nucl,Pero |
| Vc42g36.8 | VaccDscaff42-processed-36.8 | 232 | 25.4 | 5.16 | 46.05 | -0.316 | Nucl,Pero |
| Vc19g135.9 | VaccDscaff19-processed-135.9 | 222 | 23.7 | 7.08 | 55.23 | -0.605 | Nucl,Pero |
| Vc30g280.2 | VaccDscaff30-processed-280.2 | 232 | 25.5 | 5.16 | 46.41 | -0.319 | Nucl |
| Vc27g295.0 | VaccDscaff27-processed-295.0 | 263 | 28.8 | 8.80 | 62.35 | -0.655 | Nucl,Pero |
| Vc17g324.14 | VaccDscaff17-processed-324.14 | 263 | 28.8 | 8.59 | 62.70 | -0.664 | Nucl,Pero |
| Vc34g49.12 | VaccDscaff34-processed-49.12 | 263 | 28.8 | 8.59 | 62.70 | -0.664 | Nucl,Pero |
| Vc44g170.19 | VaccDscaff44-augustus-170.19 | 248 | 27.0 | 7.14 | 60.50 | -0.597 | Nucl |
| Vc20g156.15 | VaccDscaff20-augustus-156.15 | 248 | 27.0 | 6.50 | 57.33 | -0.583 | Nucl |
| Vc28g167.32 | VaccDscaff28-augustus-167.32 | 248 | 27.0 | 6.75 | 60.99 | -0.583 | Nucl |
| Vc44g65.1 | VaccDscaff44-processed-65.1 | 247 | 26.6 | 8.38 | 54.41 | -0.681 | Nucl |
| Vc28g73.2 | VaccDscaff28-processed-73.2 | 247 | 26.6 | 8.38 | 53.87 | -0.681 | Nucl |
| Vc20g302.3 | VaccDscaff20-processed-302.3 | 250 | 26.9 | 7.80 | 55.74 | -0.688 | Nucl |
| Vc8g145.9 | VaccDscaff8-processed-145.9 | 237 | 25.8 | 7.87 | 57.57 | -0.784 | Nucl |

图4 VcC2H2-ZFP在蓝莓不同器官、不同发育阶段果实和花芽休眠解除过程中的表达谱
Fig. 4 Expression profiles of VcC2H2-ZFP in diverse organs,fruit development,and the process of flower bud dormancy release
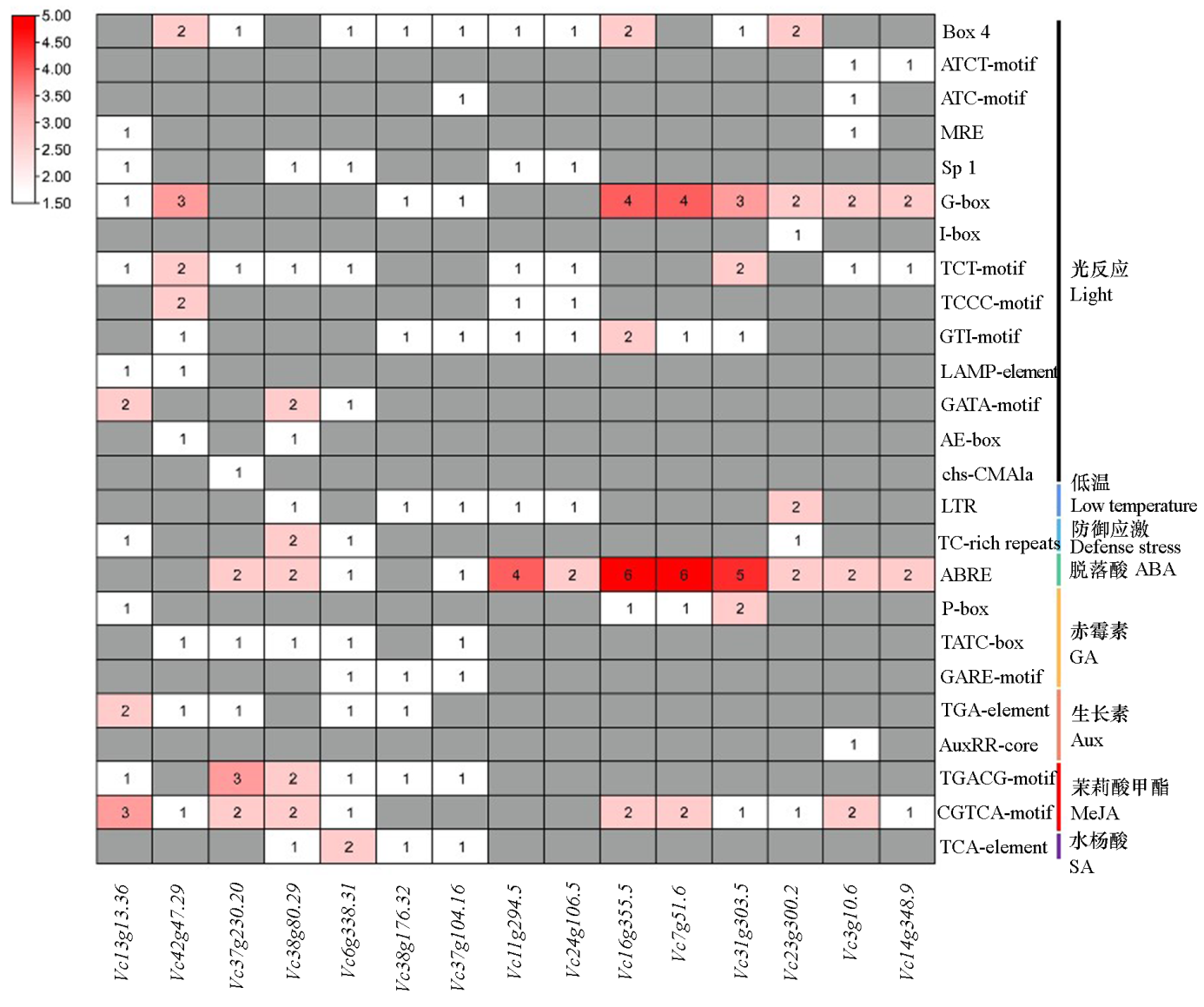
图7 VcC2H2-ZFP启动子顺式作用元件分析 图中的数字代表启动子中所包含的相应元件数量。
Fig. 7 Analysis of cis-acting elements of VcC2H2-ZFP promoter The numbers in the heat map represent the quantity of corresponding elements contained in the promoter.
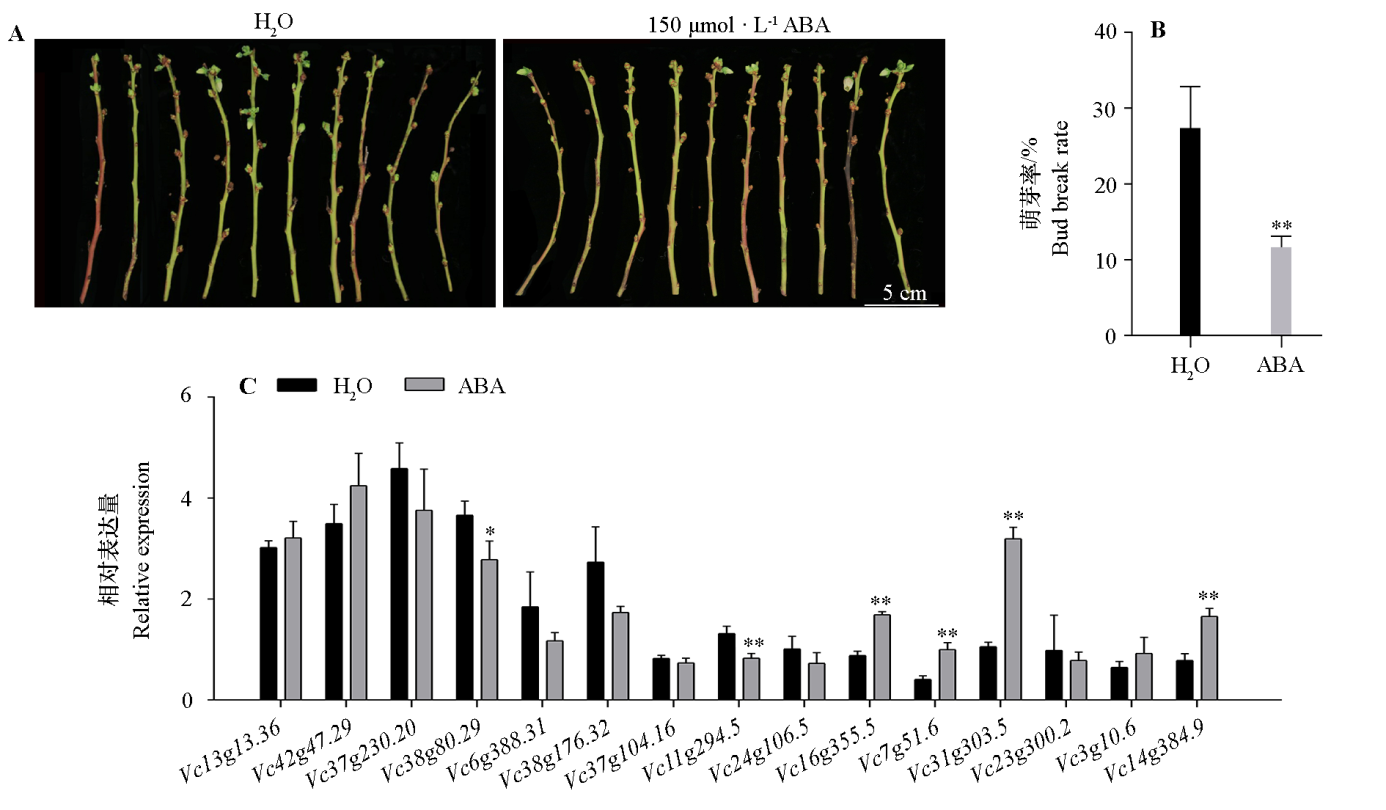
图8 外源ABA处理后蓝莓花芽萌芽率及VcC2H2-ZFP响应 *表示P < 0.05水平有显著性差异,**表示P < 0.01水平有显著性差异。
Fig. 8 Break percentage of blueberry flower bud and response of VcC2H2-ZFP after exogenous ABA treatment * indicated the significant difference under P < 0.05,** indicated the significant difference under P < 0.01.
| [1] |
Akhtar A, Becker P B. 2001. The histone H 4 acetyltransferase MOF uses a C2HC zinc finger for substrate recognition. EMBO Reports, 2 (2):113-118.
pmid: 11258702 |
| [2] | Alam I, Batool K, Cui D L, Yang Y Q, Lu Y H. 2019. Comprehensive genomic survey,structural classification and expression analysis of C2H2 zinc finger protein gene family in Brassica rapa L. PLoS ONE, 14 (5):e0216071. |
| [3] | An Shuang, Gao Yu-di, MaidinuerÝusupu, Pan Yi-na, Shao Wan, Zong Yu, Chen Wen-rong, Yang Li, Guo Wei-dong, Li Yong-qiang. 2020. Research on application exogenous abscisic acid in inhibiting blueberry early flowering and associated genes expression characteristics. Journal of Fruit Science, 38 (3):325-334. (in Chinese) |
| 安爽, 高玉迪, 麦迪努尔·玉苏普, 潘益娜, 邵婉, 宗宇, 陈文荣, 杨莉, 郭卫东, 李永强. 2020. 外源脱落酸抑制蓝莓早花及相关基因表达特性研究. 果树学报, 38 (3):325-334. | |
| [4] |
Arora R, Rowland L J, Tanino K J H. 2003. Induction and release of bud dormancy in woody perennials:a science comes of age. HortScience, 38 (5):911-921.
doi: 10.21273/HORTSCI.38.5.911 URL |
| [5] |
Bailey T L, Johnson J, Grant C E, Noble W S. 2015. The MEME Suite. Nucleic Acids Research, 43 (W1):W39-W49.
doi: 10.1093/nar/gkv416 URL |
| [6] |
Berg J M. 1988. Proposed structure for the zinc-binding domains from transcription factor IIIA and related proteins. Proc Natl Acad Sci U S A, 85 (1):99-102.
doi: 10.1073/pnas.85.1.99 URL |
| [7] |
Chen C, Chen H, Zhang Y, Thomas H R, Frank M H, He Y, Xia R. 2020a. TBtools:an integrative toolkit developed for interactive analyses of big biological data. Molecular Plant, 13 (8):1194-1202.
doi: 10.1016/j.molp.2020.06.009 URL |
| [8] |
Chen Y, Wang G, Pan J, Wen H, Du H, Sun J, Zhang K, Lv D, He H, Cai R, Pan J. 2020b. Comprehensive genomic analysis and expression profiling of the C2H2zinc finger protein family under abiotic stresses in cucumber(Cucumis sativus L.). Genes, 11 (2):171.
doi: 10.3390/genes11020171 URL |
| [9] |
Ciftci Y S, Mittler R. 2008. The zinc finger network of plants. Cellular and Molecular Life Sciences, 65:1150-1160.
doi: 10.1007/s00018-007-7473-4 pmid: 18193167 |
| [10] |
Ciftci Y S, Morsy M R, Song L, Coutu A, Krizek B A, Lewis M W, Warren D, Cushman J, Connolly E L, Mittler R. 2007. The EAR-motif of the Cys2/His2-type zinc finger protein Zat 7 plays a key role in the defense response of Arabidopsis to salinity stress. The Journal of Biological Chemistry, 282 (12):9260-9268.
doi: 10.1074/jbc.M611093200 URL |
| [11] | Colle M, Leisner C P, Wai C M, Ou S, Bird K A, Wang J, Wisecaver J H, Yocca A E, Alger E I, Tang H, Xiong Z, Callow P, Ben-Zvi G, Brodt A, Baruch K, Swale T, Shiue L, Song G Q, Childs K L, Schilmiller A, Vorsa N, Buell C R, van Buren R, Jiang N, Edger P P. 2019. Haplotype-phased genome and evolution of phytonutrient pathways of tetraploid blueberry. Gigascience, 8 (3):giz012. |
| [12] |
Davletova S, Schlauc K, Coutu J, Mittler R. 2005. The zinc-finger protein Zat 12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiology, 139 (2):847-856.
doi: 10.1104/pp.105.068254 pmid: 16183833 |
| [13] |
Englbrecht C C, Schoof H, Bohm S. 2004. Conservation,diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genomics, 5 (1):39.
doi: 10.1186/1471-2164-5-39 pmid: 15236668 |
| [14] |
Gotz K P, Naher J, Fettke J, Chmielewski F M. 2018. Changes of proteins during dormancy and bud development of sweet cherry(Prunus avium L.). Science Horticulturae, 239:41-49.
doi: 10.1016/j.scienta.2018.05.016 URL |
| [15] |
Gourcilleau D, Lenne C, Armenise C, Moulia B, Julien J L, Bronner G, Leblanc-Fournier N. 2011. Phylogenetic study of plant Q-type C2H2 zinc finger proteins and expression analysis of poplar genes in response to osmotic,cold and mechanical stresses. DNA Research, 18 (2):77-92.
doi: 10.1093/dnares/dsr001 pmid: 21367962 |
| [16] |
Han G, Wang M, Yuan F, Sui N, Song J, Wang B. 2014. The CCCH zinc finger protein gene AtZFP1 improves salt resistance in Arabidopsis thaliana. Plant Molecular Biology, 86 (3):237-253.
doi: 10.1007/s11103-014-0226-5 URL |
| [17] | Horton P, Park K J, Obayashi T, Fujita N, Harada H, Adams-Collier C J, Nakai K. 2007. WoLF PSORT:protein localization predictor. Nucleic Acids Research(Web Server Issue):W585-W587. |
| [18] |
Horvath D P, Anderson J V, Chao W S, Foley M E. 2003. Knowing when to grow:signals regulating bud dormancy. Trends in Plant Science, 8 (11):534-540.
doi: 10.1016/j.tplants.2003.09.013 pmid: 14607098 |
| [19] |
Hu X, Zhu L, Zhang Y, Xu L, Li N, Zhang X, Pan Y. 2019. Genome-wide identification of C2H2 zinc-finger genes and their expression patterns under heat stress in tomato(Solanum lycopersicum L.). PeerJ, 7:e7929.
doi: 10.7717/peerj.7929 URL |
| [20] | Jing Jian-ling, Zhang Peng, Wang Zhen-yu, Ma Qiu-xiang. 2020. Genome-wide identification and expression analysis of C2H2-type zinc finger protein transcription factor family in cassava. Plant Physiology Journal, 56 (12):2664-2676. (in Chinese) |
| 井建玲, 张鹏, 王振宇, 马秋香. 2020. 木薯C2H2型锌指蛋白转录因子家族全基因组鉴定及表达分析. 植物生理学报, 56 (12):2664-2676. | |
| [21] |
Kagale S, Links M G, Rozwadowski K. 2010. Genome-wide analysis of ethylene-responsive element binding factor-associated amphiphilic repression motif-containing transcriptional regulators in Arabidopsis. Plant Physiology, 152 (3):1109-1134.
doi: 10.1104/pp.109.151704 URL |
| [22] |
Kazan K. 2006. Negative regulation of defence and stress genes by EAR-motif-containing repressors. Trends in Plant Science, 11 (3):109-112.
doi: 10.1016/j.tplants.2006.01.004 pmid: 16473545 |
| [23] |
Klug A. 2010. The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annual Review of Biochemistry, 79:213-231.
doi: 10.1146/annurev-biochem-010909-095056 pmid: 20192761 |
| [24] |
Kubo K, Sakamoto A, Kobayashi A, Rybka Z, Kanno Y, Nakagawa H, Takatsuji H. 1998. Cys2/His2 zinc-finger protein family of petunia:evolution and general mechanism of target-sequence recognition. Nucleic Acids Research, 26 (2):608-615.
doi: 10.1093/nar/26.2.608 pmid: 9421523 |
| [25] |
Lang G A, Early J D, Martin G C, Darnell R L. 1987. Endo-,para-,and ecodormancy:physiological terminology and classification for dormancy research. HortScience, 22 (3):371-377.
doi: 10.21273/HORTSCI.22.3.371 URL |
| [26] | Li Ya-dong, Pei Jia-bo, Chen Li, Sun Hai-yue. 2021. China blueberry industry report 2020. Journal of Jilin Agricultural University, 43 (1):1-8. (in Chinese) |
| 李亚东, 裴嘉博, 陈丽, 孙海悦. 2021. 2020中国蓝莓产业年度报告. 吉林农业大学学报, 43 (1):1-8. | |
| [27] |
Li Y Q, Ma R, Li R X, Zhao Q, Zhang Z Z, Zong Y, Yao L B, Chen W R, Yang L, Liao F L, Zhu Y Y, Guo W D. 2022. Comparative transcriptomic analysis provides insight into the key regulatory pathways and differentially expressed genes in blueberry flower bud endo- and ecodormancy release. Horticulturae, 8:176.
doi: 10.3390/horticulturae8020176 URL |
| [28] | Liu Q, Wang Z, Xu X, Zhang H, Li C. 2015. Genome-wide analysis of C2H2zinc-finger family transcription factors and their responses to abiotic stresses in poplar(Populus trichocarpa). PLoS ONE, 10 (8):e0134753. |
| [29] |
Liu Z, Coulter J A, Li Y, Zhang X, Meng J, Zhang J, Liu Y. 2020. Genome-wide identification and analysis of the Q-type C2H2 gene family in potato (Solanum tuberosum L.). International Journal of Biological Macromolecules, 153:327-340.
doi: 10.1016/j.ijbiomac.2020.03.022 URL |
| [30] |
Mackay J P, Crossley M. 1998. Zinc fingers are sticking together. Trends in Biochemical Sciences, 23 (1):1-4.
pmid: 9478126 |
| [31] |
Miller J, McLachlan A D, Klug A. 1985. Repetitive zinc-binding domains in the protein transcription factor Ⅲ A from Xenopus oocytes. The EMBO Journal, 4 (6):1609-1614.
doi: 10.1002/embj.1985.4.issue-6 URL |
| [32] |
Noman A, Aqeel M, Khalid N, Islam W, Sanaullah T, Anwar M, Khan S, Ye W, Lou Y. 2019. Zinc finger protein transcription factors:integrated line of action for plant antimicrobial activity. Microbial Pathogenesis, 132:141-149.
doi: S0882-4010(18)31546-8 pmid: 31051192 |
| [33] |
Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M. 2001. Repression domains of classⅡERF transcriptional repressors share an essential motif for active repression. The Plant Cell, 13 (8):1959-1968.
doi: 10.1105/TPC.010127 URL |
| [34] |
Saitou N, Nei M. 1987. The neighbor-joining method:a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4 (4):406-425.
doi: 10.1093/oxfordjournals.molbev.a040454 pmid: 3447015 |
| [35] |
Sakamoto H, Maruyama K, Sakuma Y, Meshi T, Iwabuchi M, Shinozaki K, Yamaguchi-Shinozaki K. 2004. Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought,cold,and high-salinity stress conditions. Plant Physiology, 136 (1):2734-2746.
doi: 10.1104/pp.104.046599 pmid: 15333755 |
| [36] |
Samish R M. 1954. Dormancy in woody plants. Annual Review of Plant Physiology, 5 (1):183-204.
doi: 10.1146/arplant.1954.5.issue-1 URL |
| [37] |
Sanchez-Garcia I, Rabbitts T H. 1994. The LIM domain:a new structural motif found in zinc-finger-like proteins. Trends in Genetics, 10 (9):315-320.
doi: 10.1016/0168-9525(94)90034-5 URL |
| [38] | Song Yang, Liu Hongdi, Wang Haibo, Zhang Hongjun, Liu Fengzhi. 2019. Molecular cloning and functional characterization of anthocyanin synthesis related genes VcTTG1of blueberry. Acta Horticulturae Sinica, 46 (7):1270-1278. (in Chinese) |
|
宋杨, 刘红弟, 王海波, 张红军, 刘凤之. 2019. 越橘花青苷合成相关基因VcTTG1的克隆与功能鉴定. 园艺学报, 46 (7):1270-1278.
doi: 10.16420/j.issn.0513-353x.2018-0768 |
|
| [39] | Wu Yue-yan, Li Bo, Zhu Ping, Hu Hua-yong. 2011. Effects of plant growth regulator on flowering and endogenous hormones of Rhododendron hybridum. Acta Horticulturae Sinica, 38 (8):1565-1571. (in Chinese) |
| 吴月燕, 李波, 朱平, 胡华勇. 2011. 植物生长调节剂对西洋杜鹃花期及内源激素的影响. 园艺学报, 38 (4):337-349. | |
| [40] |
Yang Q S, Gao Y H, Wu X Y, Moriguchi T, Bai S L, Teng Y W. 2021. Bud endodormancy in deciduous fruit trees:advances and prospects. Horticulture Research, 8 (1):139.
doi: 10.1038/s41438-021-00575-2 |
| [41] | Yang Yu-chun, Wei Xin, Sun Bin, Zhang Duo, Wang Xing-dong, Liu You-chun, Wei Yong-xiang, Tian Ying, Liu Cheng. 2020. Research analysis of cold requirement of different blueberry varieties at low temperature. Bulletin of Agricultural Science and Technology,(1):178-181. (in Chinese) |
| 杨玉春, 魏鑫, 孙斌, 张舵, 王兴东, 刘有春, 魏永祥, 田颖, 刘成. 2020. 蓝莓不同品种低温需冷量研究分析. 农业科技通讯,(1):178-181. | |
| [42] | Yang Yu-chun, Wei Yong-xiang, Wei Xin, Liu You-chun, Wang Xing-dong, Sun Bin, Zhang Duo, Liu Cheng. 2021. Dynamic changes of antioxidant enzyme activities and endogenous hormone contents during flower bud dormancy of two blueberry cultivars. China Fruits,(8):31-35,42. (in Chinese) |
| 杨玉春, 魏永祥, 魏鑫, 刘有春, 王兴东, 孙斌, 张舵, 刘成. 2021. 2个蓝莓品种花芽休眠期抗氧化酶活性及内源激素含量动态变化分析. 中国果树,(8):31-35,42. | |
| [43] |
Yao Fuwen, Wang Meige, Song Chunhui, Song Shangwei, Jiao Jian, Wang Miaomiao, Wang Kun, Bai Tuanhui, Zheng Xianbo. 2021. Identification and expression analysis of HSP90 gene family under high temperature stress in apple. Acta Horticulturae Sinica, 48 (5):849-859. (in Chinese)
doi: 10.16420/j.issn.0513-353x.2020-0895 |
|
姚富文, 王枚阁, 宋春晖, 宋尚伟, 焦健, 王苗苗, 王昆, 白团辉, 郑先波. 2021. 苹果HSP90家族基因鉴定及高温胁迫下的表达分析. 园艺学报, 48 (5):849-859.
doi: 10.16420/j.issn.0513-353x.2020-0895 |
|
| [44] | Yu Ke-da, Ye Mei-juan, Chen Wen-rong, Zhu Kai-li, Zhang Chang-jing, Guo Wei-dong. 2016. Methods for RNA isolation from blueberry tissues. Journal of Zhejiang Normal University(Nat Sci), 39 (1):60-64. (in Chinese) |
| 余柯达, 叶美娟, 陈文荣, 朱凯丽, 张常晶, 郭卫东. 2016. 蓝莓组织RNA提取方法的研究. 浙江师范大学学报(自然科学版), 39 (1):60-64. | |
| [45] |
Yu Lei, Zhou Ya, Zong Yu, Zhang Ying, Qiu Jiaqi, Li Yongqiang, Yang Li, Guo Weidong. 2021. Characteristic and relative expression pattern analysis of FWL/PLAC 8 family in blueberry. Acta Horticulturae Sinica, 48 (2):336-346. (in Chinese)
doi: 10.16420/j.issn.0513-353x.2020-0151 |
|
俞蕾, 周雅, 宗宇, 张颖, 邱佳琪, 李永强, 杨莉, 郭卫东. 2021. 越橘FWL/PLAC8家族基因特征及表达分析. 园艺学报, 48 (2):336-346.
doi: 10.16420/j.issn.0513-353x.2020-0151 URL |
|
| [46] |
Zhang Huilin, Zhu Wan, Tian Li, Zhang Wei. 2019. Characterization and expression analysis of petunia PhZPT2- 12 transcription factor related to cold response. Acta Horticulturae Sinica, 46 (8):1543-1552. (in Chinese)
doi: 10.16420/j.issn.0513-353x.2018-0891 |
|
张慧琳, 朱婉, 田丽, 张蔚. 2019. 矮牵牛冷响应转录因子PhZPT2-12的特性及表达分析. 园艺学报, 46 (8):1543-1552.
doi: 10.16420/j.issn.0513-353x.2018-0891 URL |
| [1] | 饶智雄, 安玉艳, 曹荣祥, 唐泉, 汪良驹. 外源ALA缓解ABA抑制草莓根系伸长生长的机理研究[J]. 园艺学报, 2023, 50(3): 461-474. |
| [2] | 王晓晨, 聂子页, 刘先菊, 段伟, 范培格, 梁振昌. 脱落酸对‘京香玉’葡萄果实单萜物质合成的影响[J]. 园艺学报, 2023, 50(2): 237-249. |
| [3] | 洪 坡, 朱东姿, 王甲威, 张力思, 孙 山, 刘庆忠. 兔眼蓝莓新品种‘蓝冠’[J]. 园艺学报, 2022, 49(S2): 43-44. |
| [4] | 梁沁, 张延晖, 康开权, 刘瑾航, 李亮, 冯宇, 王超, 杨超, 李永裕. miR168家族进化特性及其在砂梨休眠期的表达模式分析[J]. 园艺学报, 2022, 49(5): 958-972. |
| [5] | 许曈, 邵灵梅, 王小斌, 张润龙, 张凯靖, 夏宜平, 张佳平, 李丹青. 多年生单子叶植物的越冬休眠研究进展[J]. 园艺学报, 2022, 49(12): 2703-2721. |
| [6] | 侯天泽, 易双双, 张志群, 王健, 李崇晖. 秋石斛RT-qPCR内参基因的筛选与验证[J]. 园艺学报, 2022, 49(11): 2489-2501. |
| [7] | 于建强, 顾凯迪, 王传增, 胡大刚. 苹果磷酸果糖激酶基因MdPFPβ调控果实可溶性糖积累的功能[J]. 园艺学报, 2022, 49(10): 2223-2235. |
| [8] | 杨博, 魏佳, 李坤峰, 王程亮, 倪隽蓓, 滕元文, 白松龄. PpyERF060-PpyABF3-PpyMADS71调控乙烯信号通路介导的梨芽休眠进程[J]. 园艺学报, 2022, 49(10): 2249-2262. |
| [9] | 徐国辉, 安 琪, 赵丽娜, 刘国玲, 娄 鑫, 王贺新, . 适合穗状采收的蓝莓新品种‘晨雪’[J]. 园艺学报, 2021, 48(S2): 2795-2796. |
| [10] | 徐国辉, 安 琪, 刘国玲, 赵丽娜, 王贺新, . 蓝莓新品种‘逐梦’[J]. 园艺学报, 2021, 48(S2): 2797-2798. |
| [11] | 杨为海, 曾利珍, 肖秋生, 石胜友. 饥饿胁迫下龙眼落果与果皮和离区糖、ABA及相关基因表达的变化[J]. 园艺学报, 2021, 48(8): 1457-1469. |
| [12] | 曾泽湘, 肖显梅, 谭小丽, 范中奇, 陈建业. 菜薹BrWRKY57的特性及其对BrPPH1和BrNCED3的调控[J]. 园艺学报, 2021, 48(3): 518-530. |
| [13] | 李钰卓, 刘柯, 袁璐, 曹丽雯, 王挺进, 甘苏生, 陈利萍. 菜薹BrNAP克隆及其在采后叶片衰老中的功能分析[J]. 园艺学报, 2021, 48(1): 60-72. |
| [14] | 乔永刚,曹亚萍,贾孟君,王勇飞,贺嘉欣,张鑫瑞,王文斌,宋 芸*. 连翘异型花柱植株花芽生长发育与传粉习性研究[J]. 园艺学报, 2020, 47(4): 699-707. |
| [15] | 戴文珊 1,2,王 敏 1,2,刘继红 1,*. 柠檬中超表达印度酸橘脱落酸合成基因 CrNCED1增强脱水抗性[J]. 园艺学报, 2020, 47(3): 551-561. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司