
园艺学报 ›› 2022, Vol. 49 ›› Issue (3): 687-700.doi: 10.16420/j.issn.0513-353x.2021-0636
收稿日期:2021-09-23
修回日期:2022-03-01
出版日期:2022-03-25
发布日期:2022-03-25
通讯作者:
王雁
E-mail:wyancaf@163.com
基金资助:
ZHOU Lin, ZOU Hongzhu, HAN Lulu, JIA Yinghua, WANG Yan*( )
)
Received:2021-09-23
Revised:2022-03-01
Online:2022-03-25
Published:2022-03-25
Contact:
WANG Yan
E-mail:wyancaf@163.com
摘要:
糖基转移酶属于催化糖基转移反应的超基因家族的一类酶。糖基化修饰能丰富花青素的结构,调节其水溶性、稳定性和可转运性,决定植物色泽形成。对参与观赏植物花色素成分合成代谢的糖基转移酶的种类及其生化、分子生物学研究进行梳理,并对其在蓝紫色、橙色–紫红色以及黄色等主要花瓣色泽形成中的作用进行归纳,展望色泽相关糖基转移酶的研究方向,以期为深入解析花色素物质的糖基化修饰机理提供参考,也为观赏植物花色改良提供新思路。
中图分类号:
周琳, 邹红竹, 韩璐璐, 贾莹华, 王雁. 糖基转移酶在花瓣色泽形成中的作用研究进展[J]. 园艺学报, 2022, 49(3): 687-700.
ZHOU Lin, ZOU Hongzhu, HAN Lulu, JIA Yinghua, WANG Yan. Research Progress on the Role of Glycosyltransferases in Color Formation of Petals[J]. Acta Horticulturae Sinica, 2022, 49(3): 687-700.
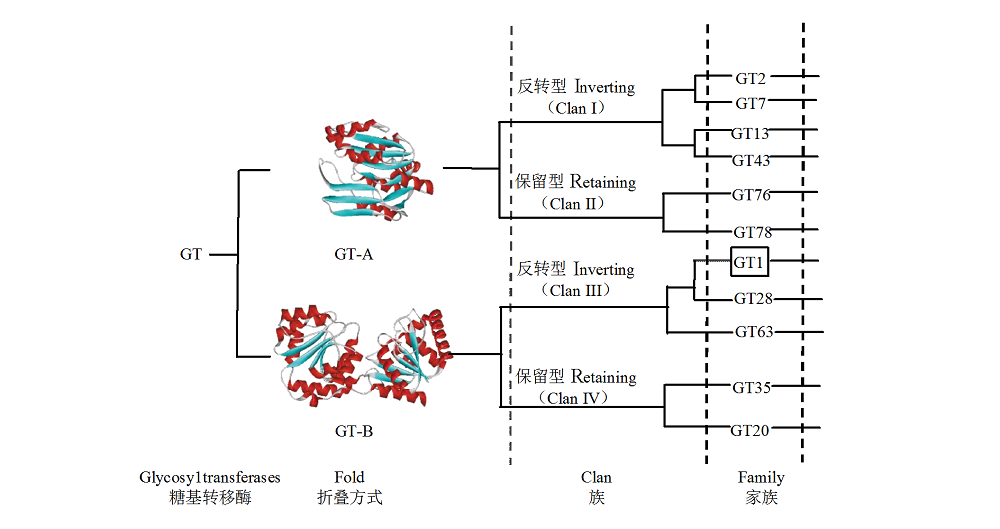
图1 糖基转移酶从折叠方式到族和家族的等级分类 方框内为色泽形成相关糖基转移酶所属的GT1家族。
Fig. 1 The hierarchical classification of glycosyltransferases from folds to clans,and families The box shows the GT1 family that glycosyltransferases related to color formation belong to.
| 花色类型 Flower color type | 物种 Species | 糖基转移酶 GT | 登录号 Acession number | 底物 Acceptor substrate | 糖供体 Donor substrate | 产物 Product |
|---|---|---|---|---|---|---|
| 蓝紫色 Blue-violet | 三花龙胆1 Gentiana triflora | 3'GT | AB076697 | 飞燕草素3,5-O-二葡萄糖苷Delphinidin 3,5-O-diglucoside | UDP-葡萄糖 UDP-Glc | 飞燕草素3,5,3'-O-三葡萄糖苷Delphinidin 3,5,3'-O- triglucoside |
| Gt5GT7 | AB363839 | 飞燕草素3-O-葡萄糖苷 Delphinidin 3-O-diglucoside | UDP-葡萄糖 UDP-Glc | 飞燕草素3,5-O-二葡萄糖苷 Delphinidin 3,5-O-diglucoside | ||
| 蝶豆2 Clitoria ternatea | UA3'5'GT | _ | 飞燕草素3-O-(6''-O-丙二酰基)-β-葡萄糖苷 Delphinidin 3-O-(6"-O- malonyl)-β-glucoside | UDP-葡萄糖 UDP-Glc | 飞燕草素 3-O-(6''-O-丙二酰基)-β-葡萄糖基-3', 5'-双-O-β-葡萄糖苷Delphinidin 3-O-(6"-O-malonyl)-β-glucoside-3',5'-di-O-β-glucoside | |
| 婆婆纳3 Veronica persica | UGT88D8 | AB465708 | 芹菜素 Apigenin | UDP-葡萄糖醛酸 UDP-glucuronate | 芹菜素7-O-(2-O-葡萄糖醛酸基)-葡萄糖醛酸苷Apigenin 7-O-(2-O-glucuronosyl)- glucuronide | |
| UGT94F1 | AB514127 | 飞燕草素3-O-葡萄糖苷 Delphinidin 3-O-glucoside | UDP-葡萄糖 UDP-Glc | 飞燕草素3-O-槐糖苷Delphinidin 3-O-sophoroside | ||
| 矮牵牛4 Petunia hybrida | PH1 | AB027455 | 飞燕草素3-(p-酰基)-芸香糖苷Delphinidin 3-(p-coumaroyl)-rutinoside | UDP-葡萄糖 UDP-Glc | 飞燕草素3-(p-酰基)-芸香糖基-5-O-葡萄糖苷 Delphinidin 3-(p-coumaroyl)- rutinoside-5-O-glucoside | |
| 六倍利5 Lobelia erinus | ABRT2,ABRT4 | BAU68118,BAU68119 | 飞燕草素3-O-葡萄糖苷 Delphinidin 3-O-glucoside | UDP-鼠李糖UDP-rhamnose | 飞燕草素3-O-芸香糖苷Delphinidin 3-O-rutinoside | |
| 紫花鸢尾6 Iris ensata | 5GT | _ | 花青素3-O-鼠李糖基葡萄糖苷Anthocyanidin 3-O-rhamnosylglucoside | UDP-葡萄糖 UDP-Glc | 花青素3-O-鼠李糖基葡萄糖苷-5-O-葡萄糖苷Anthocyanidin 3-O-rhamnosylglucoside-5-O- glucoside | |
| 飞燕草7 Delphinium grandiflorum | AA7GT | AB510758 | 飞燕草素-3-O-芸香糖苷Delphinidin 3-O-rutinoside | 对羟基苯甲酰基葡萄糖p-hydroxybenzoyl-Glc | 飞燕草素3-O-芸香糖苷-7- O-葡萄糖苷Delphinidin 3-O-rutinoside-7-O-glucoside | |
| AA7BG-GT1,AA7BG-GT2 | AB811444,AB811447 | 飞燕草素3-O-芸香苷-7-O-(6-O-[对羟基苯甲酰基]-葡萄糖苷)Delphinidin 3-O-rutinoside- 7-O-(6-O-[p-hydroxybenzoyl]-glucoside) | 对羟基苯甲酰基葡萄糖p-hydroxybenzoyl-Glc | 飞燕草素3-O-芸香苷-7-O-(6-O-(4-O-(葡萄糖基)-氧苯甲酰基)-葡萄糖苷 Delphinidin 3-O-rutinoside- 7-O-(6-O-(4-O-(glucosyl)-oxybenzoyl)-glucoside | ||
| 百子莲8 Agapanthus africanus | AaAA7GT | AB692769 | 花青素3-O-葡萄糖苷 Anthocyanidin 3-O-glycosides | 酰基葡萄糖acyl-glucose | 花青素3,7-O-二葡萄糖苷 Anthocyanidin 3,7-O-diglucoside | |
| 橙色—紫红色Orange- | 美女樱9 Verbena hybrida | 5GT | AB013598 | 矢车菊素3-O-葡萄糖苷 Cyanidin 3-O-glucoside | UDP-葡萄糖 UDP-Glc | 矢车菊素3,5-O-二葡萄糖苷 Cyanidin 3,5-O-diglucoside |
| purplish red | 牵牛花10 Ipomoea purpurea | 3GT | LC019110 | 矢车菊素 Cyanidin | UDP-葡萄糖 UDP-Glc | 矢车菊素3-O-葡萄糖苷 Cyanidin 3-O-glucoside |
| 玫瑰11 Rosa hybrida | RhGT1 | AB201048 | 矢车菊素、矢车菊素5- O-葡萄糖苷 Cyanidin,Cyanidin 5-O-glucoside | UDP-葡萄糖 UDP-Glc | 矢车菊素5-O-葡萄糖苷, 矢车菊素3,5-O-葡萄糖苷 Cyanidin 5-O-glucoside,Cyanidin 3,5-O-glucoside | |
| 小苍兰12 Freesia hybrid | Fh3GT1 | HM590645 | 矢车菊素 Cyanidin | UDP-葡萄糖 UDP-Glc | 矢车菊素3-O-葡萄糖苷 Cyanidin 3-O-glucoside | |
| 雏菊13 Bellis perennis | BpUGAT | AB190262 | 矢车菊素3-O-6''-O-丙二酰葡糖苷Cyanidin 3-O-6''-O-malonylglucoside | UDP-葡萄糖醛酸UDP-glucuronate | 矢车菊素3-O-2''-O-β-葡糖醛酸基-6''-O-丙二酰葡萄糖苷Cyanidin 3-O-2''-O-β- glucuronosyl-6''-O-malonylglucoside | |
| 花色类型 Flower color type | 物种 Species | 糖基转移酶 GT | 登录号 Acession number | 底物 Acceptor substrate | 糖供体 Donor substrate | 产物 Product |
| 香石竹14 Dianthus caryophyllus | AA5GT | AB646510 | 天竺葵素3-葡萄糖苷 Pelargonidin 3-O-glucoside | 酰基葡萄糖acyl-glucose | 天竺葵素3,5-O-二葡萄糖苷 Pelargonidin 3,5-O-diglucoside | |
| 岩桐15 Sinningia ccardinalis | dA5GT | AB537182 | 木犀草素、芹菜素Luteolinidin,apigeninidin | UDP-葡萄糖 UDP-Glc | 木犀草素5-O-葡萄糖苷, 芹菜素5-O-葡萄糖苷 Luteolinidin 5-O-glucoside,apigeninidin 5-O-glucoside | |
| 彩虹菊16 Dorotheanthus bellidiformis | Betanidin 5GT,betanidin 6GT | Y18871,AF374004 | 甜菜苷元 Betanidin | UDP-葡萄糖 UDP-Glc | 甜菜红素 Betacyanin | |
| 紫茉莉17 Mirabilis jalapa | MjcDOPA5GT | AB182643 | 环多巴 cyclo-DOPA | UDP-葡萄糖 UDP-Glc | 环多巴5-O-葡萄糖苷 cDOPA 5-O-glucoside | |
| 鸡冠花18 Celosia cristata | CccDOPA5GT | AB182644 | 环多巴 cyclo-DOPA | UDP-葡萄糖 UDP-Glc | 环多巴5-O-葡萄糖苷 cDOPA 5-O-glucoside | |
| 黄色 Yellow | 香石竹19 Dianthus caryophyllus | DicGT4,DicGT5 | AB191248,AB191249 | 查耳酮 Chalcone | UDP-葡萄糖 UDP-Glc | 查耳酮2'-O-糖苷 Chalcone 2'-O-glucoside |
| 金鱼草20 Antirrhinum majus | Am4'CGT | AB198665 | 查耳酮 Chalcone | UDP-葡萄糖 UDP-Glc | 查耳酮4'-O-糖苷 Chalcone 4'-O-glucoside | |
| 番红花21 Crocus sativus | UGTCs2,UGTCs3 | AY262037,AY290820 | 西红花酸 Crocetin | UDP-葡萄糖 UDP-Glc | 西红花苷 Crocin |
表1 观赏植物花瓣色泽形成相关糖基转移酶
Table 1 Glycosyltransferases related to petal color formation in ornamental plants
| 花色类型 Flower color type | 物种 Species | 糖基转移酶 GT | 登录号 Acession number | 底物 Acceptor substrate | 糖供体 Donor substrate | 产物 Product |
|---|---|---|---|---|---|---|
| 蓝紫色 Blue-violet | 三花龙胆1 Gentiana triflora | 3'GT | AB076697 | 飞燕草素3,5-O-二葡萄糖苷Delphinidin 3,5-O-diglucoside | UDP-葡萄糖 UDP-Glc | 飞燕草素3,5,3'-O-三葡萄糖苷Delphinidin 3,5,3'-O- triglucoside |
| Gt5GT7 | AB363839 | 飞燕草素3-O-葡萄糖苷 Delphinidin 3-O-diglucoside | UDP-葡萄糖 UDP-Glc | 飞燕草素3,5-O-二葡萄糖苷 Delphinidin 3,5-O-diglucoside | ||
| 蝶豆2 Clitoria ternatea | UA3'5'GT | _ | 飞燕草素3-O-(6''-O-丙二酰基)-β-葡萄糖苷 Delphinidin 3-O-(6"-O- malonyl)-β-glucoside | UDP-葡萄糖 UDP-Glc | 飞燕草素 3-O-(6''-O-丙二酰基)-β-葡萄糖基-3', 5'-双-O-β-葡萄糖苷Delphinidin 3-O-(6"-O-malonyl)-β-glucoside-3',5'-di-O-β-glucoside | |
| 婆婆纳3 Veronica persica | UGT88D8 | AB465708 | 芹菜素 Apigenin | UDP-葡萄糖醛酸 UDP-glucuronate | 芹菜素7-O-(2-O-葡萄糖醛酸基)-葡萄糖醛酸苷Apigenin 7-O-(2-O-glucuronosyl)- glucuronide | |
| UGT94F1 | AB514127 | 飞燕草素3-O-葡萄糖苷 Delphinidin 3-O-glucoside | UDP-葡萄糖 UDP-Glc | 飞燕草素3-O-槐糖苷Delphinidin 3-O-sophoroside | ||
| 矮牵牛4 Petunia hybrida | PH1 | AB027455 | 飞燕草素3-(p-酰基)-芸香糖苷Delphinidin 3-(p-coumaroyl)-rutinoside | UDP-葡萄糖 UDP-Glc | 飞燕草素3-(p-酰基)-芸香糖基-5-O-葡萄糖苷 Delphinidin 3-(p-coumaroyl)- rutinoside-5-O-glucoside | |
| 六倍利5 Lobelia erinus | ABRT2,ABRT4 | BAU68118,BAU68119 | 飞燕草素3-O-葡萄糖苷 Delphinidin 3-O-glucoside | UDP-鼠李糖UDP-rhamnose | 飞燕草素3-O-芸香糖苷Delphinidin 3-O-rutinoside | |
| 紫花鸢尾6 Iris ensata | 5GT | _ | 花青素3-O-鼠李糖基葡萄糖苷Anthocyanidin 3-O-rhamnosylglucoside | UDP-葡萄糖 UDP-Glc | 花青素3-O-鼠李糖基葡萄糖苷-5-O-葡萄糖苷Anthocyanidin 3-O-rhamnosylglucoside-5-O- glucoside | |
| 飞燕草7 Delphinium grandiflorum | AA7GT | AB510758 | 飞燕草素-3-O-芸香糖苷Delphinidin 3-O-rutinoside | 对羟基苯甲酰基葡萄糖p-hydroxybenzoyl-Glc | 飞燕草素3-O-芸香糖苷-7- O-葡萄糖苷Delphinidin 3-O-rutinoside-7-O-glucoside | |
| AA7BG-GT1,AA7BG-GT2 | AB811444,AB811447 | 飞燕草素3-O-芸香苷-7-O-(6-O-[对羟基苯甲酰基]-葡萄糖苷)Delphinidin 3-O-rutinoside- 7-O-(6-O-[p-hydroxybenzoyl]-glucoside) | 对羟基苯甲酰基葡萄糖p-hydroxybenzoyl-Glc | 飞燕草素3-O-芸香苷-7-O-(6-O-(4-O-(葡萄糖基)-氧苯甲酰基)-葡萄糖苷 Delphinidin 3-O-rutinoside- 7-O-(6-O-(4-O-(glucosyl)-oxybenzoyl)-glucoside | ||
| 百子莲8 Agapanthus africanus | AaAA7GT | AB692769 | 花青素3-O-葡萄糖苷 Anthocyanidin 3-O-glycosides | 酰基葡萄糖acyl-glucose | 花青素3,7-O-二葡萄糖苷 Anthocyanidin 3,7-O-diglucoside | |
| 橙色—紫红色Orange- | 美女樱9 Verbena hybrida | 5GT | AB013598 | 矢车菊素3-O-葡萄糖苷 Cyanidin 3-O-glucoside | UDP-葡萄糖 UDP-Glc | 矢车菊素3,5-O-二葡萄糖苷 Cyanidin 3,5-O-diglucoside |
| purplish red | 牵牛花10 Ipomoea purpurea | 3GT | LC019110 | 矢车菊素 Cyanidin | UDP-葡萄糖 UDP-Glc | 矢车菊素3-O-葡萄糖苷 Cyanidin 3-O-glucoside |
| 玫瑰11 Rosa hybrida | RhGT1 | AB201048 | 矢车菊素、矢车菊素5- O-葡萄糖苷 Cyanidin,Cyanidin 5-O-glucoside | UDP-葡萄糖 UDP-Glc | 矢车菊素5-O-葡萄糖苷, 矢车菊素3,5-O-葡萄糖苷 Cyanidin 5-O-glucoside,Cyanidin 3,5-O-glucoside | |
| 小苍兰12 Freesia hybrid | Fh3GT1 | HM590645 | 矢车菊素 Cyanidin | UDP-葡萄糖 UDP-Glc | 矢车菊素3-O-葡萄糖苷 Cyanidin 3-O-glucoside | |
| 雏菊13 Bellis perennis | BpUGAT | AB190262 | 矢车菊素3-O-6''-O-丙二酰葡糖苷Cyanidin 3-O-6''-O-malonylglucoside | UDP-葡萄糖醛酸UDP-glucuronate | 矢车菊素3-O-2''-O-β-葡糖醛酸基-6''-O-丙二酰葡萄糖苷Cyanidin 3-O-2''-O-β- glucuronosyl-6''-O-malonylglucoside | |
| 花色类型 Flower color type | 物种 Species | 糖基转移酶 GT | 登录号 Acession number | 底物 Acceptor substrate | 糖供体 Donor substrate | 产物 Product |
| 香石竹14 Dianthus caryophyllus | AA5GT | AB646510 | 天竺葵素3-葡萄糖苷 Pelargonidin 3-O-glucoside | 酰基葡萄糖acyl-glucose | 天竺葵素3,5-O-二葡萄糖苷 Pelargonidin 3,5-O-diglucoside | |
| 岩桐15 Sinningia ccardinalis | dA5GT | AB537182 | 木犀草素、芹菜素Luteolinidin,apigeninidin | UDP-葡萄糖 UDP-Glc | 木犀草素5-O-葡萄糖苷, 芹菜素5-O-葡萄糖苷 Luteolinidin 5-O-glucoside,apigeninidin 5-O-glucoside | |
| 彩虹菊16 Dorotheanthus bellidiformis | Betanidin 5GT,betanidin 6GT | Y18871,AF374004 | 甜菜苷元 Betanidin | UDP-葡萄糖 UDP-Glc | 甜菜红素 Betacyanin | |
| 紫茉莉17 Mirabilis jalapa | MjcDOPA5GT | AB182643 | 环多巴 cyclo-DOPA | UDP-葡萄糖 UDP-Glc | 环多巴5-O-葡萄糖苷 cDOPA 5-O-glucoside | |
| 鸡冠花18 Celosia cristata | CccDOPA5GT | AB182644 | 环多巴 cyclo-DOPA | UDP-葡萄糖 UDP-Glc | 环多巴5-O-葡萄糖苷 cDOPA 5-O-glucoside | |
| 黄色 Yellow | 香石竹19 Dianthus caryophyllus | DicGT4,DicGT5 | AB191248,AB191249 | 查耳酮 Chalcone | UDP-葡萄糖 UDP-Glc | 查耳酮2'-O-糖苷 Chalcone 2'-O-glucoside |
| 金鱼草20 Antirrhinum majus | Am4'CGT | AB198665 | 查耳酮 Chalcone | UDP-葡萄糖 UDP-Glc | 查耳酮4'-O-糖苷 Chalcone 4'-O-glucoside | |
| 番红花21 Crocus sativus | UGTCs2,UGTCs3 | AY262037,AY290820 | 西红花酸 Crocetin | UDP-葡萄糖 UDP-Glc | 西红花苷 Crocin |
| [1] | Ahrazem O, Rubio-Moraga A, Jimeno M L, Gómez-Gómez L. 2015. Structural characterization of highly glucosylated crocins and regulation of their biosynthesis during flower development in Crocus. Frontiers in Plant Science, 6:971. |
| [2] |
Bourne Y, Henrissat B. 2001. Glycoside hydrolases and glycosyltransferases:families and functional modules. Current Opinion in Structural Biology, 11(5):593-600.
pmid: 11785761 |
| [3] |
Brugliera F, Holton A, Stevenson T W, Farcy E, Lu C Y, Cornish E. 1994. Isolation and characterization of a cDNA clone corresponding to the Rt locus of Petunia hybrida. The Plant Journal, 5:81-92.
doi: 10.1046/j.1365-313X.1994.5010081.x URL |
| [4] |
Campbell J A, Davies G J, Bulone V, Henrissat B. 1997. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochemical Journal, 326:929-939.
doi: 10.1042/bj3260929u URL |
| [5] |
Castañeda-Ovando A, Pacheco-Hernández M D L, Páez-Hernández M E, Rodríguez J A, Galán-Vidal C A. 2009. Chemical studies of anthocyanins:a review. Food Chemistry, 113:859-871.
doi: 10.1016/j.foodchem.2008.09.001 URL |
| [6] |
Castillo R, Fernandez J A, Gomez-Gomez L. 2005. Implications of carotenoid biosynthetic genes in apocarotenoid formation during the stigma development of Crocus sativus and its closer relatives. Plant Physiology, 139:674-689.
pmid: 16183835 |
| [7] |
Clifford M N. 2000. Anthocyanins-nature occurrence and dietary burden. Journal of the Science of Food and Agriculture, 80:1063-1072.
doi: 10.1002/(ISSN)1097-0010 URL |
| [8] |
Coutinho P M, Deleury E, Davies G J, Henrissat B. 2003. An evolving hierarchical family classification for glycosyltransferases. Journal of Molecular Biology, 328(2):307-317.
pmid: 12691742 |
| [9] | Dai Si-lan, Hong Yan. 2016. Molecular breeding for flower colors modification on ornamental plants based on the mechanism of anthocyanins biosynthesis and coloration. Scientia Agricultura Sinica, 49(3):529-542. |
| 戴思兰, 洪艳. 2016. 基于花青素苷合成和呈色机理的观赏植物花色改良分子育种. 中国农业科学, 49(3):529-542. | |
| [10] |
Dufresne C, Cormier F, Dorion S. 1997. In vitro formation of crocetin glucosyl esters by Crocus sativus callus extract. Planta Medica, 63:150-153.
doi: 10.1055/s-2006-957633 pmid: 9140230 |
| [11] |
Feng C Y, Li S S, Taguchi G, Wu Q, Yin D D, Gu Z Y, Wu J, Xu W Z, Liu C, Wang L S. 2021. Enzymatic basis for stepwise C-glycosylation in the formation of flavonoid di-C-glycosides in sacred lotus(Nelumbo nucifera Gaertn.). The Plant Journal, 106(2):351-365.
doi: 10.1111/tpj.v106.2 URL |
| [12] |
Forkmann G. 1991. Flavonoids as flower pigments:the formation of the natural spectrum and its extension by genetic engineering. Plant Breed, 106:1-26.
doi: 10.1111/pbr.1991.106.issue-1 URL |
| [13] |
Fossen T, Andersen O M. 1999. Delphinidin 3′-galloyl-galactosides from blue flowers of Nymphaea caerulea. Phytochemistry, 50:1185-1188.
doi: 10.1016/S0031-9422(98)00649-9 URL |
| [14] | Fukuchi-Mizutani M, Okuhara H, Fukui Y, Nakao M, Katsumoto Y. 2003. Biochemical and molecular characterization of a novel UDP-glucose:anthocyanin 3'-O-glucosyltransferase,a key enzyme for blue anthocyanin biosynthesis from gentian. Plant Physiology, 32:1652-1663. |
| [15] |
Harborne J B. 1966. Comparative biochemistry of flavonoids,II:3-desoxyanthocyanins and their systematic distribution in ferns and gesnerads. Phytochemistry, 5:589-600.
doi: 10.1016/S0031-9422(00)83637-7 URL |
| [16] | Hashimoto F, Tanaka M, Maeda H, Fukuda S, Shimizu K, Sakata Y. 2002. Changes in flower coloration and sepal anthocyanins of cyanic delphinium cultivars during flowering. Journal of the Agricultural Chemical Society of Japan, 66:1652-1659. |
| [17] |
He J B, Zhao P, Hu Z M, Liu S, Kuang Y, Zhang M, Li B, Yun C H, Qiao X, Ye M. 2019. Molecular and structural characterization of a promiscuous C-glycosyltransferase from Trollius chinensis. Angewandte Chemie-international Edition, 58:11513-11520.
doi: 10.1002/anie.v58.33 URL |
| [18] |
Hedin P A, Lamar P L, Thompson A C, Minyard J P. 1968. Isolation and structural determination of 13 flavonoid glycosides in Hibiscus esculentus (Okra). American Journal of Botany, 55(4):431-437.
pmid: 5641298 |
| [19] |
Hiromoto T, Honjo E, Noda N, Tamada T, Kazuma K, Suzuki M, Blaber M, Kuroki R. 2015. Structural basis for acceptor-substrate recognition of UDP-glucose:anthocyanidin 3-O-glucosyltransferase from Clitoria ternatea. Protein Science, 24:395-407.
doi: 10.1002/pro.2630 pmid: 25556637 |
| [20] |
Honda T, Saito N. 2002. Recent progress in the chemistry of polyacylated anthocyanins as flower color pigments. Heterocycles, 56:633-692.
doi: 10.3987/REV-01-SR(K)2 URL |
| [21] |
Hsu Y H, Tagami T, Matsunaga K, Okuyama M, Suzuki T, Noda N, Suzuki M, Shimura H. 2017. Functional characterization of UDP-rhamnose-dependent rhamnosyltransferase involved in anthocyanin modification,a key enzyme determining blue coloration in Lobelia erinus. The Plant Journal, 89(2):325-337.
doi: 10.1111/tpj.13387 URL |
| [22] |
Hu Y, Walker S. 2002. Remarkable structural similarities between diverse glycosyltransferases. Chemistry & Biology, 9:1287-1296.
doi: 10.1016/S1074-5521(02)00295-8 URL |
| [23] | Huang Jin-xia, Wang Liang-sheng, Li Xiao-mei, Lu Ying-qing. 2006. Advances in molecular basis and evolution of floral color variation. Chinese Bulletin of Botany, 23(4):321-333. (in Chinese) |
| 黄金霞, 王亮生, 李晓梅, 鲁迎青. 2006. 花色变异的分子基础与进化模式研究进展. 植物学通报, 23(4):321-333. | |
| [24] |
Imayama T, Yoshihara N, Fukuchi-Mizutani M, Tanaka Y, Ino I, Yabuya T. 2004. Isolation and characterization of a cDNA clone of UDP-glucose:anthocyanin 5-O-glucosyltransferase in Iris hollandica. Plant Science, 167:1243-1248.
doi: 10.1016/j.plantsci.2004.06.020 URL |
| [25] |
Ishii I, Sakaguchi K, Fujita K, Fujita K, Ozeki Y, Miyahara T. 2017. A double knockout mutant of acyl-glucose-dependent anthocyanin glucosyltransferase genes in Delphinium grandiflorum. Journal of Plant Physiology, 216:74.
doi: 10.1016/j.jplph.2017.05.009 URL |
| [26] |
Iwashina T, Takemura T, Mishio T. 2009. Chalcone glycoside in the flowers of six Corylopsis species as yellow pigment. Journal of the Japanese Society for Horticultural Science, 78:485-490.
doi: 10.2503/jjshs1.78.485 URL |
| [27] |
Jones P, Vogt T. 2001. Glycosyltransferases in secondary plant metabolism:tranquilizers and stimulant controllers. Planta, 213(2):164-174.
pmid: 11469580 |
| [28] |
Ju Z, Sun W, Meng X, Liang L, Li Y, Zhou T, Shen H, Xiang G, Wang L. 2018. Isolation and functional characterization of two 5-O-glucosyltransferases related to anthocyanin biosynthesis from Freesia hybrid. Plant Cell Tissue and Organ Culture, 135:99-110.
doi: 10.1007/s11240-018-1447-0 URL |
| [29] |
Kang X, Mikami R, Akita Y. 2021. Characterization of 5-O-glucosyltransferase involved in anthocyanin biosynthesis in Cyclamen purpurascens. Plant Biotechnology, 38(2):263-268.
doi: 10.5511/plantbiotechnology.21.0308a URL |
| [30] |
Kogawa K, Kato N, Kazuma K, Noda N, Suzuki M. 2007. Purification and characterization of UDP-glucose:anthocyanin 3',5'-O-glucosyltransferase from Clitoria ternatea. Planta, 226(6):1501-1509.
doi: 10.1007/s00425-007-0584-1 URL |
| [31] |
Kong J M, Chia L S, Goh N K, Chia T F, Brouillard R. 2003. Analysis and biological activities of anthocyanins. Phytochemistry, 64(5):923-933.
doi: 10.1016/S0031-9422(03)00438-2 URL |
| [32] |
Kroon J, Souer E, Graaff A D, Xue Y, Koes R. 1994. Cloningand structural analysis of the anthocyanin pigmentation locus Rt of Petunia hybrida:characterization of insertion sequences in two mutant alleles. The Plant Journal, 5:69-80.
doi: 10.1046/j.1365-313X.1994.5010069.x URL |
| [33] |
Kuhn B, Forkman G, Seyffert W. 1978. Genetic control ofchalcone-flavanone isomerase activity in Callistephus chinensis. Planta, 138:199-203.
doi: 10.1007/BF00386811 pmid: 24414046 |
| [34] |
Lairson L L, Henrissat B, Davies G J, Withers S G. 2008. Glycosyltransferases:structures,functions,and mechanism. Annual Review of Biochemistry, 77:521-557.
doi: 10.1146/annurev.biochem.76.061005.092322 pmid: 18518825 |
| [35] |
Lepiniec L, Debeaujon I, Routaboul J M, Baudry A, Pourcel L, Nesi N, Caboche M. 2006. Genetics and biochemistry of seed flavonoids. Annual Review of Plant Biology, 57:405-430.
pmid: 16669768 |
| [36] |
Li Y, Baldauf S, Lim E K, Bowles D J. 2001. Phylogenetic analysis of the UDP-glycosyltransferasemultigene family of Arabidopsis thaliana. The Journal of Biological Chemistry, 276(6):4338-4343.
doi: 10.1074/jbc.M007447200 URL |
| [37] |
Liao Y H, Houghton P J, Hoult J R. 1999. Novel and known constituents from Buddleja species and their activity against leukocyte eicosanoid generation. Journal of Natural Products, 62:1241-1245.
pmid: 10514305 |
| [38] | Mackenzie, Owens I S, Burchell B, Bock K W, Bairoch A, Belanger A, Gigleux S F, Green M, Hum D W, Iyanagi T. 1997. The UDP glycosyltransferase gene superfamily:recommended nomenclature update based on evolutionary divergence. Pharmacogenetics & Genomics, 7:255-269. |
| [39] | Malik V, Black G W. 2012. Structural,functional,and mutagenesis studies of UDP-glycosyltransferases. Advances in Protein Chemistry and Structural Biology, 87:87-115. |
| [40] | Masada S, Terasaka K, Oguchi Y, Okazaki S, Mizushima T, Mizukami H. 2009. Functional and structural characterization of a flavonoid glucoside 1,6-glucosyltransferase from Catharanthus roseus. Plant & Cell Physiology, 50:1401-1415. |
| [41] |
Matsuba Y, Sasaki N, Tera M, Okamura M, Abe Y, Okamoto E. 2010. A novel glucosylation reaction on anthocyanins catalyzed by acylglucose-dependent glucosyltransferase in the petals of carnation and delphinium. Plant Cell, 22:3374-3389.
doi: 10.1105/tpc.110.077487 URL |
| [42] |
Miyahara T, Takahashi M, Ozeki Y, Sasaki N. 2012. Isolation of an acyl-glucose-dependent anthocyanin 7-O-glucosyltransferase from the monocot Agapanthus africanus. Journal of Plant Physiology, 169:1321-1326.
doi: 10.1016/j.jplph.2012.05.004 pmid: 22673029 |
| [43] | Miyajima I, Maehara T, Kage T, Fujieda K. 1991. Identification of the main agent causing yellow color of yellow-flowered cyclamen mutant. Journal of the Japanese Society for Horticultural Science, 60:409-414. |
| [44] |
Mizuno T, Yabuya T, Kitajima J, Iwashina T. 2013. Identification of novel C-glycosylflavones and their contribution to flower colour of the Dutch iris cultivars. Plant Physiology and Biochemistry, 72:116-124.
doi: 10.1016/j.plaphy.2013.06.028 URL |
| [45] |
Montefiori M, Espley R V, Stevenson D, Cooney J, Allan A C. 2011. Identification and characterisation of F3GT1 and F3GGT1,two glycosyltransferases responsible for anthocyanin biosynthesis inred-fleshed kiwifruit(Actinidia chinensis). The Plant Journal, 65:106-118.
doi: 10.1111/j.1365-313X.2010.04409.x pmid: 21175894 |
| [46] |
Moraga A R, Nohales P F, Perez J A, Gomez-Gomez L. 2004. Glucosylation of the saffron apocarotenoid crocetin by a glucosyltransferase isolatedfrom Crocus sativus stigmas. Planta, 219:955-966.
doi: 10.1007/s00425-004-1299-1 URL |
| [47] |
Morita Y, Ishiguro K, Tanaka Y, Iida S, Hoshino A. 2015. Spontaneous mutations of the UDP-glucose:flavonoid 3-O-glucosyltransferase gene confers pale- and dull-colored flowers in the Japanese and common morning glories. Planta, 242:575-587.
doi: 10.1007/s00425-015-2321-5 URL |
| [48] |
Nakatsuka T, Nishihara M, Mishiba K, Yamamura S. 2005. Temporal expression of flavonoid biosynthesis-related genesregulates flower pigmentation in gentian plants. Plant Science, 168:1309-1318.
doi: 10.1016/j.plantsci.2005.01.009 URL |
| [49] |
Nakatsuka T, Sato K, Takahashi H, Yamamura S, Nishihara M. 2008. Cloning and characterization of the UDP-glucose:anthocyanin 5-O-glucosyltransferase gene from blue-flowered gentian. Journal of Experimental Botany, 59:1241-1252.
doi: 10.1093/jxb/ern031 pmid: 18375606 |
| [50] |
Nakatsuka T, Nishihara M. 2010. UDP-glucose:3-deoxyanthocyanidin 5-O-glucosyltransferase from Sinningia cardinalis. Planta, 232:383-392.
doi: 10.1007/s00425-010-1175-0 pmid: 20458497 |
| [51] |
Nakayama T. 2002. Enzymology of aurone biosynthesis. Journal of Bioscience and Bioengineering, 94(6):487-491.
pmid: 16233339 |
| [52] |
Nishizaki Y, Matsuba Y, Okamoto E, Okamura M, Ozeki Y, Sasaki N. 2011. Structure of the acyl-glucose-dependent anthocyanin 5-O-glucosyltransferase gene in carnations and its disruption by transposable elements in some varieties. Molecular Genetics and Genomics, 286:383-394.
doi: 10.1007/s00438-011-0655-7 pmid: 22048706 |
| [53] |
Nishizaki Y, Sasaki N, Yasunaga M, Miyahara T, Okamoto E, Okamoto M, Hirose Y, Ozeki Y. 2014. Identification of the glucosyltransferase gene that supplies the p-hydroxybenzoyl-glucose for 7-polyacylation of anthocyanin in delphinium. Journal of Experimental Botany, 65(9):2495-2506.
doi: 10.1093/jxb/eru134 pmid: 24723398 |
| [54] |
Nishizaki Y, Yasunaga M, Okamoto E, Okamoto M, Hirose Y, Yamaguchi M, Ozeki Y, Sasaki N. 2013. p-Hydroxybenzoyl-glucose is a zwitter donor for the biosynthesis of 7-polyacylated anthocyanin in delphinium. Plant Cell, 25:4150-4165.
doi: 10.1105/tpc.113.113167 URL |
| [55] |
Noguchi A, Horikawa M, Fukui Y, Fukuchi-Mizutani M, IuchiOkada A, Ishiguro M, Kiso Y, Nakayama T, Ono E. 2009. Local differentiation of sugar donor specificity of flavonoid glycosyltransferase in Lamiales. Plant Cell, 21:1556-1572.
doi: 10.1105/tpc.108.063826 pmid: 19454730 |
| [56] |
Ogata J, Itoh Y, Ishida M, Yoshida H, Ozeki Y. 2004. Cloning and heterologous expression of a cDNA encoding flavonoid glucosyltransferases from Dianthus caryophyllus. Plant Biotechnology, 21:367-375.
doi: 10.5511/plantbiotechnology.21.367 URL |
| [57] |
Ogata J, Sakamoto T, Yamaguchi M, Kawanobu S, Yoshitama K. 2001. Isolation and characterization of anthocyanin 5-O-glucosyltransferase from flowers of Dahlia variabilis. Journal of Plant Physiology, 158:709-714.
doi: 10.1078/0176-1617-00370 URL |
| [58] |
Ogata J, Kanno Y, Itoh Y, Tsugawa H, Suzuki M. 2005. Plant biochemistry:anthocyanin biosynthesis in roses. Nature, 435(7043):757-758.
doi: 10.1038/nature435757a URL |
| [59] |
Ono E, Ruike M, Iwashita T, Nomoto K, Fukui Y. 2010. Co-pigmentation and flavonoid glycosyltransferases in blue Veronica persica flowers. Phytochemistry, 71(7):726-735.
doi: 10.1016/j.phytochem.2010.02.008 URL |
| [60] | Ono E, Fukuchi-Mizutani M, Nakamura N, Fukui Y, Yonekura-Sakakibara K, Yamaguchi M, Nakayama T, Tanaka T, Kusumi T, Tanaka Y. 2006. Yellow flowers generated by expression of the aurone biosynthetic pathway. Proceedings of the National Academy of Sciences, 103(29):11075-11080. |
| [61] |
Peterson J, Dwyer J. 1998. Flavonoids:dietary occurrence and biochemical activity. Nutrition Research, 18(12):1995-2018.
doi: 10.1016/S0271-5317(98)00169-9 URL |
| [62] |
Polturak G, Breitel D, Grossman N, Sarrion-Perdigones A, Weithorn E, PlinerM, Orzaez D, Granell A, Rogachev I, Aharoni A. 2016. Elucidation of the first committed step in betalain biosynthesis enables the heterologous engineering of betalain pigments in plants. New Phytologist, 210:269-283.
doi: 10.1111/nph.13796 pmid: 26683006 |
| [63] | Polturak G, Grossman N, Vela-Corcia D, Dong Y, Nudel A, Pliner M, Levy M, Rogachev I, Aharoni A. 2017. Engineered gray mold resistance,antioxidant capacity,and pigmentation in betalain-producing crops and ornamentals. Proceedings of the National Academy of Sciences, 114(34):9062-9067. |
| [64] |
Prior R L, Wu X. 2006. Anthocyanins:structural characteristics that result in unique metabolic patterns and biological activities. Free Radical Research, 40:1014-1028.
doi: 10.1080/10715760600758522 URL |
| [65] |
Rogachev I, Aharoni A. 2016. Elucidation of the first committed step in betalain biosynthesis enables the heterologous engineering of betalain pigments in plants. New Phytologist, 210:269-283.
doi: 10.1111/nph.2016.210.issue-1 URL |
| [66] |
Ross J, Yi L, Lim E K, Bowles D J. 2001. Higher plant glycosyltransferases. Genome Biology, 2 (2):3004.1-3004.6.
pmid: 11182895 |
| [67] | Rubio Moraga A, Ahrazem O, Rambla J L, Granell A, Gómez Gómez L. 2013. Crocins with high levels of sugar conjugation contribute to the yellow colours of early-spring flowering Crocus tepals. PLoS ONE, 8:e71946. |
| [68] |
Saito K, Yonekura-Sakakibara K, Nakabayashi R, Higashi Y, Yamazaki M, Tohge T, Fernie A R. 2013. The flavonoid biosynthetic pathway in Arabidopsis:structural and genetic diversity. Plant Physiology and Biochemistry, 72:21-34.
doi: 10.1016/j.plaphy.2013.02.001 URL |
| [69] |
Saito N, Tatsuzawa F, Miyoshi K, Shigihara A, Honda T. 2003. The first isolation of C-glycosylanthocyanin from the flowers of Tricyrtis formosana. Tetrahedron Letters, 44:6821-6823.
doi: 10.1016/S0040-4039(03)01747-7 URL |
| [70] |
Santos-Buelga C, Mateus N, Freitas V D. 2014. Anthocyanins. Plant pigments and beyond. Journal of Agriculture and Food Chemistry, 62:6879-6884.
doi: 10.1021/jf501950s URL |
| [71] |
Sasaki N, Adachi T, Goda Y, Ozeki Y. 2004. Detection of UDP-glucose:cyclo-DOPA 5-O-glucosyltransferase activity in four o’clocks(Mirabilis jalapa L.). FEBS Letters, 568:159-162.
doi: 10.1016/j.febslet.2004.04.097 URL |
| [72] |
Sasaki N, Abe Y, Wada K, Koda T, Goda Y, Adachi T, Ozeki Y. 2005a. Amaranthin in feather cockscombs is synthesized via glucuronylation at the cyclo-DOPA glucoside step in the betacyanin biosynthetic pathway. Journal of Plant Research, 118:439-442.
doi: 10.1007/s10265-005-0237-z URL |
| [73] | Sasaki N, Wada K, Koda T, Kasahara K, Adachi T, Ozeki Y. 2005b. Isolation and characterization of cDNAs encoding anenzyme with glucosyltransferase activity for cyclo-DOPA from four o’clocks and feather cockscombs. Plant & Cell Physiology, 46:666-670. |
| [74] |
Sawada S, Suzuki H, Ichimaida F, Yamaguchi M A, Iwashita T, Fukui Y, Hemmi H, Nishino T, Nakayama T. 2005. UDP-glucuronic acid:anthocyanin glucuronosyltransferase from red daisy(Bellis perennis)flowers. Enzymology and phylogenetics of a novel glucuronosyltransferase involved in flower pigment biosynthesis. Journal of Biological Chemistry, 280:899-906.
doi: 10.1074/jbc.M410537200 URL |
| [75] |
Shao H, He X, Achnine L, Blount J W, Dixon R A, Wang X. 2005. Crystal structures of a multifunctional triterpene flavonoidglycosyltransferase from Medicago truncatula. The Plant Cell, 17:3141-3154.
doi: 10.1105/tpc.105.035055 URL |
| [76] |
Strack D, Vogt T, Schliemann W. 2003. Recent advances in betalain research. Phytochemistry, 62:247-269.
doi: 10.1016/S0031-9422(02)00564-2 URL |
| [77] |
Sui X, Gao X, Ao M, Wang Q, Yang D, Wang M, Yang F, Wang L. 2011. cDNA cloning and characterization of UDP-glucose:anthocyanidin 3-O-glucosyltransferase in Freesia hybrid. Plant Cell Reports, 30:1209-1218.
doi: 10.1007/s00299-011-1029-7 URL |
| [78] | Sun W, Liang L, Meng X, Li Y, Gao F, Liu X, Wang S, Gao X, Wang L. 2016. Biochemical and molecular characterization of a flavonoid 3-O-glycosyltransferase responsible for anthocyanins and flavonols biosynthesis in Freesia hybrida. Frontiers in Plant Science, 7:410. |
| [79] | Tanaka Y, Brugliera F. 2013. Flower colour and cytochromes P450. Philosophical Transactions of the Royal Society of London,Series B, 368:20120432. |
| [80] |
Tanaka Y, Katsumoto Y, Brugliera F, Mason J. 2005. Genetic engineering in floriculture. Plant Cell Tissue and Organ Culture, 80:1-24.
doi: 10.1007/s11240-004-0739-8 URL |
| [81] | Tanaka Y, Sasaki N, Ohmiya A. 2008. Biosynthesis of plant pigments:anthocyanins,betalains and carotenoids. The Plant Jouranl, 54:733-749. |
| [82] | Tanaka Y, Yonekura K, Fukuchi-Mizutani M, Fukui Y, Fujiwara H, Ashikari T, Kusumi T. 1996. Molecular and biochemical characterization of three anthocyanin synthetic enzymes from Gentiana triflora. Plant & Cell Physiology, 37:711-716. |
| [83] | Tatsuzawa F, Saito N, Miyoshi K, Shinoda K, Shigihara A, Honda T. 2004. Diacylated 8-C-glucosylcyanidin 3-glucoside from the flowers of Tricyrtis formosana. Chemical & Pharmaceutical Bulletin, 52:631-633. |
| [84] |
Timoneda A, Feng T, Sheehan H, Walker-Hale N, Pucker B, Lopez-Nieves S, Guo R, Brockington S. 2019. The evolution of betalain biosynthesis in Caryophyllales. The New Phytologist, 224(1):71-85.
doi: 10.1111/nph.v224.1 URL |
| [85] |
Timoneda A, Sheehan H, Feng T, Lopez-Nieves S, Maeda H A, Brockington S. 2018. Redirecting primary metabolism to boost production of tyrosine-derived specialised metabolites in planta. Scientific Reports, 8:17256.
doi: 10.1038/s41598-018-33742-y pmid: 30467357 |
| [86] |
Tiwari P, Sangwan R, Sangwan S. 2016. Plant secondary metabolism linked glycosyltransferases:an update on expanding knowledge and scopes. Biotechnology Advances, 34(5):714-739.
doi: 10.1016/j.biotechadv.2016.03.006 URL |
| [87] | Togami J, Okuhara H, Nakamura N, Ishiguro K, Hirose C, Ochiai M, Fukui Y, Yamaguchi M, Tanaka Y. 2011. Isolation of cDNAs encoding tetrahydroxychalcone 2'-glucosyltransferase activity from carnation,cyclamen,and catharanthus. Plant Tissue Culture Letters, 28(2):231-238. |
| [88] | Trošt K, Golc-Wondra A, Prošek M, Milivojevič L. 2008. Anthocyanin degradation of blueberry-aronia nectar in glass compared with carton during storage. Journal Food Science, 73:405-411. |
| [89] |
Vogt T. 2002. Substrate specificity and sequence analysis define a polyphyletic origin of betanidin 5- and 6-O-glucosyltransferase from Dorotheanthus bellidiformis. Planta, 214:492-495.
doi: 10.1007/s00425-001-0685-1 URL |
| [90] |
Vogt T, Grimm R, Strack D. 2002. Cloning and expression of a cDNA encoding betanidin 5-O-glucosyltransferase,a betanidin- and flavonoid-specific enzyme with high homology to inducible glucosyltransferases from the Solanaceae. The Plant Journal, 19:509-519.
doi: 10.1046/j.1365-313X.1999.00540.x URL |
| [91] |
Vogt T, Jones P. 2000. Glycosyltransferases in plant natural product synthesis:characterization of a super gene family. Trends in Plant Science, 5(9):380-386.
pmid: 10973093 |
| [92] | Xiao J. 2017. Dietary flavonoid aglycones and their glycosides:which show better biological significance? Critical Reviews in Food Science and Nutrition, 57(9):1874-1905. |
| [93] | Xie Linfeng, Ren Chuanhong, Zhang Bo, Xu Changjie, Li Xian. 2019. Plant UDP-glycosyltransferases in flavonoids biosynthesis. Acta Horticulturae Sinica, 46(9):1655-1669. (in Chinese) |
| 解林峰, 任传宏, 张波, 徐昌杰, 李鲜. 2019. 植物类黄酮生物合成相关UDP-糖基转移酶研究进展. 园艺学报, 46(9):1655-1669. | |
| [94] | Yabuya T, Yamaguchi M, Imayama T, Katoh K, Ino I. 2002. Anthocyanin 5-O-glucosyltransferase in flowers of Iris ensata. Plant Science, 262:779-784. |
| [95] |
Yamazaki M, Gong Z, Fukuchi-Mizutani M, Fukui Y, Tanaka Y, Kusumi T, Saito K. 1999. Molecular cloning and biochemical characterization of a novel anthocyanin 5-O-glucosyltransferase by mRNA differential display for plant forms regarding anthocyanin. Journal of Biological Chemistry, 274:7405-7411.
doi: 10.1074/jbc.274.11.7405 pmid: 10066805 |
| [96] |
Yamazaki M, Yamagishi E, Gong Z, Fukuchi-Mizutani M, Fukui Y, Tanaka Y, Kusumi T, Yamaguchi M, Saito K. 2002. Two flavonoid glucosyltransferases from Petunia hybrida:molecular cloning,biochemical properties and developmentally regulated expression. Plant Molecular Biology, 48:401-411.
pmid: 11905966 |
| [97] |
Yang Y, Li B, Feng C Y, Wu Q, Wang Q, Li S S, Yu X N, Wang L S. 2020. Chemical mechanism of flower color microvariation in Paeonia with yellow flowers. Horticultural Plant Journal, 6(3):179-190.
doi: 10.1016/j.hpj.2020.04.002 URL |
| [98] | Yim S H, Lee Y J, Park K D, Lee I S, Shin B A, Jung D W, Williams D R, Kim H J. 2015. Phenolic constituents from the flowers of Hamamelis japonica Sieb. et Zucc. Natural Product Sciences, 21(3):162-169. |
| [99] |
Yonekura-Sakakibara K, Fukushima A R, Nakabayashi K, Hanada F, Matsuda S, Sugawara E, Inoue T, Kuromori T, Ito K, Shinozaki B. 2012. Two glycosyltransferases involved in anthocyanin modification delineated by transcriptome independent component analysis in Arabidopsis thaliana. The Plant Journal, 69:154-167.
doi: 10.1111/j.1365-313X.2011.04779.x pmid: 21899608 |
| [100] | Yonekura-Sakakibara K, Hanada K. 2011. An evolutionary view of functional diversity in family 1 glycosyltransferases. Plant Journal for Cell & Molecular Biology, 66:182-193. |
| [101] |
Yoshida H, Itoh Y, Ozeki Y, Iwashina T, Yamaguchi M. 2004. Variation in chalcononaringenin 2'-O-glucoside contentin the petals of carnations (Dianthus caryophyllus)bearing yellow flowers. Scientia Horticulturae, 99:175-186.
doi: 10.1016/S0304-4238(03)00093-1 URL |
| [102] |
Yoshida K, Mori M, Kondo T. 2009. Blue flower color development by anthocyanins:from chemical structure to cell physiology. Natural Products Reports, 26:884-915.
doi: 10.1039/b800165k URL |
| [103] |
Zhao J. 2015. Flavonoid transport mechanisms:how to go,and with whom. Trends in Plant Science, 20:576-585.
doi: 10.1016/j.tplants.2015.06.007 URL |
| [104] |
Zhao Z X, Jin J, Lin C Z, Zhu C C, Liu Y M, Lin A H, Liu Y X, Zhang L, Luo H F. 2011. Two new chalcone glycosides from the stems of Entada phaseoloides. Fitoterapia, 82:1102-1105.
doi: 10.1016/j.fitote.2011.07.005 URL |
| [105] | Zhou Lin, Wang Yan, Lü Chun-yan, Peng Zhen-hua. 2011. Identification of components of flower pigments in petals of Paeonia lutea wild population in Yunnan. Journal of Northeast Forestry University, 39(8):52-54. (in Chinese) |
| 周琳, 王雁, 律春燕, 彭镇华. 2011. 云南野生黄牡丹花色素成分的鉴定. 东北林业大学学报, 39(8):52-54. | |
| [106] | Zhou Lin, Wang Yan, Peng Zhen-hua. 2009. Advances on formation mechanism and genetic engineering of yellow flowers. Scientia Silvae Sinicae, 45(2):111-119. (in Chinese) |
| 周琳, 王雁, 彭镇华. 2009. 黄色花形成机制及基因工程研究进展. 林业科学, 45(2):111-119. | |
| [107] |
Zhu C, Bai C, Sanahuja G, Yuan D, Farré G, Naqvi S, Shi L, Capell T, Christou P. 2010. The regulation of carotenoid pigmentation in flowers. Archives of Biochemistry and Biophysics, 504:132-141.
doi: 10.1016/j.abb.2010.07.028 URL |
| [1] | 王小斌, 张 栋, 史小华, 李丹青, 张润龙, 邵灵梅, 许 曈, 夏宜平, 张佳平, . 芍药新品种‘紫心’[J]. 园艺学报, 2022, 49(S1): 115-116. |
| [2] | 王沙, 张心慧, 赵玉洁, 李变变, 招雪晴, 沈雨, 董建梅, 苑兆和. 石榴花青苷合成相关基因PgMYB111的克隆与功能分析[J]. 园艺学报, 2022, 49(9): 1883-1894. |
| [3] | 李晓明, 于俊池, 王春夏. 露地、温室、温室遮阳下紫花和白花香青兰生长及次生代谢物比较[J]. 园艺学报, 2022, 49(6): 1363-1370. |
| [4] | 何静娟, 范燕萍. 观赏植物花色相关的类胡萝卜素组成及代谢调控研究进展[J]. 园艺学报, 2022, 49(5): 1162-1172. |
| [5] | 沈植国, 张琳, 袁德义, 程建明. 蜡梅花色及其红花新资源研究进展[J]. 园艺学报, 2022, 49(4): 924-934. |
| [6] | 王静, 徐雷锋, 王令, 祁先宇, 宋蒙, 曹雨薇, 何国仁, 唐玉超, 杨盼盼, 明军. 百合花色表型数量分类研究[J]. 园艺学报, 2022, 49(3): 571-580. |
| [7] | 卢甜甜, 刘志远, 徐兆生, 张合龙, 李国亮, 折红兵, 钱伟. 菜豆花色全基因组关联分析[J]. 园艺学报, 2022, 49(2): 332-340. |
| [8] | 邓娇, 苏梦月, 刘雪莲, 欧克芳, 户正荣, 杨平仿. 基于转录组分析揭示双色花莲‘大洒锦’花色形成机理[J]. 园艺学报, 2022, 49(2): 365-377. |
| [9] | 陈思嘉, 王焕, 李蕊蕊, 王卓异, 罗靖, 王彩云. 菊花CmMYC2在舌状花绿色性状形成过程中的功能研究[J]. 园艺学报, 2022, 49(11): 2377-2387. |
| [10] | 孙威, 孙世宇, 陈一然, 王聿晗, 张艳, 鞠志刚, 乙引. 马缨杜鹃查尔酮异构酶基因RdCHI1的克隆与功能解析[J]. 园艺学报, 2022, 49(11): 2407-2418. |
| [11] | 郭鑫, 成仿云, 钟原, 成信云, 陶熙文. 紫斑牡丹花色表型数量分类研究[J]. 园艺学报, 2022, 49(1): 86-99. |
| [12] | 张 栋, 高 聪, 林叶璠, 徐蕴晨, 刘 悦, 邱 帅, 任梓铭, 夏宜平, . 换锦花新品种‘夏梦’ [J]. 园艺学报, 2021, 48(S2): 2933-2934. |
| [13] | 张 栋, 邵灵梅, 许 曈, 申屠圆玥, 赵芳梦, 魏建芬, 夏宜平, 任梓铭, . 换锦花新品种‘含笑’[J]. 园艺学报, 2021, 48(S2): 2935-2936. |
| [14] | 谯正林, 胡慧贞, 鄢波, 陈龙清. 花香挥发性苯/苯丙素类化合物的生物合成及基因调控研究进展[J]. 园艺学报, 2021, 48(9): 1815-1826. |
| [15] | 施丽婷, 周鑫洋, 叶建丰, 周家豪, 王刚, 夏国华. 木本观赏植物远缘杂交育种研究进展[J]. 园艺学报, 2021, 48(9): 1827-1838. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2012 《园艺学报》编辑部 京ICP备10030308号-2 国际联网备案号 11010802023439
编辑部地址: 北京市海淀区中关村南大街12号中国农业科学院蔬菜花卉研究所 邮编: 100081
电话: 010-82109523 E-Mail: yuanyixuebao@126.com
技术支持:北京玛格泰克科技发展有限公司